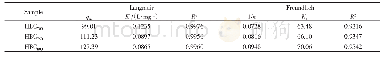
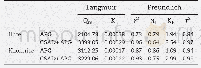
《Table 3 Adsorption isotherms parameters for the adsorption of Cu (II) and Ni (II) by AC》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Adsorption of Cu and Ni Ions from Aqueous Solutions by Commercial Activated Carbon and the Reutilization in Glass Coloration》
where Ce(mg/L),qe(mg/g),qm and KL are the concentration of analyte in solution,the amount of adsorbed analyte at equilibrium,Langmuir constants related to adsorption capacity(mg/g)and the energy of adsorption(L/g),respectively.Kf and 1/n are the Freundlich constants related to sorption capacity and sorption intensity,respectively.Comparison of the R2values in Table 3 for different isotherms shows that the Langmuir adsorption model provides the better fit by giving higher R2 values(0.997 6 and 0.999 1 for Cu (II)and Ni(II),respectively) .The results suggest that the adsorption of Cu(II)and Ni(II)ions on the AC adsorbent is monolayer adsorptions[24].
| 图表编号 | XD0043325000 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.01 |
| 作者 | 黄美苓、WANG Chao、刘世权 |
| 绘制单位 | Department of Materials Science and Engineering, University of Jinan、Department of Materials Science and Engineering, University of Jinan、Department of Materials Science and Engineering, University of Jinan |
| 更多格式 | 高清、无水印(增值服务) |