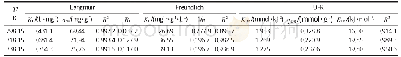
《Table 3–Fitting parameters for the adsorption isotherms of pesticide on illite and kaolinite.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Effect of an eco-friendly o/w emulsion stabilized with amphiphilic sodium alginate derivatives on lambda-cyhalothrin adsorption-desorption on natural soil minerals》
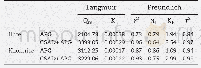
Given that the isotherm model of the adsorption process was in accordance with the Freundlich model,the desorption process was also fitted by the Freundlich model as the inverse of the adsorption process.Table 4 shows the desorption isotherm parameters of the Freundlich model for the pesticide system.A comparison of Table 3 shows that the KFdvalue of the desorption process was markedly lower than that of the adsorption process,indicating that the degree of desorption was extremely small.Moreover,the KFdvalue for the CSAD/APG system was smaller than that for the APG system in the desorption process,suggesting that the desorption of pesticide from the CSAD/APG system was weak.That is,the migration resistance of the pesticide from the soil mineral to water was large,supporting the above-mentioned fact that the pesticide adsorption capacity in the CSAD/APG system was greater than that in the APG system.In other words,the greater the adsorption capacity of soil minerals for pesticide,the weaker the migration capability of pesticide in the desorption process.
| 图表编号 | XD0033526800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.04.15 |
| 作者 | Yang Peng、Dunchao Xiao、Gaobo Yu、Yuhong Feng、Jiacheng Li、Xinyu Zhao、Yiyuan Tang、Longzheng Wang、Quan Zhang |
| 绘制单位 | Hainan University、Key Laboratory of Advanced Materials of Tropical Island Resources,Ministry of Education,College of Materials and Chemical Engineering,Hainan University、Key Laboratory of Advanced Materials of Tropical Island Resources,Ministry of Educati |
| 更多格式 | 高清、无水印(增值服务) |