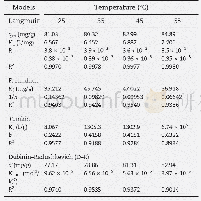
《Table 2 Adsorption isotherm parameters for Cd2+adsorption on MCM-41》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《低成本制备MCM-41介孔分子筛及其对镉离子的吸附性能(英文)》
For isotherm studies,50 m L of Cd2+solutions at different concentrations(20,30,60,80,and 100 mg/L)was equilibrated for 120 min with 130 mg MCM-41 at25°C.Fig.10 shows the experimental data and Langmuir-Freundlich isotherms for Cd2+adsorption onto MCM-41.Table 2 lists the calculated values from the Langmuir and Freundlich isothermal adsorption parameters for Cd2+adsorption.The fit of a model to the experimental data is usually evaluated by linear regression analysis,where the R2 value is used as an indication for the goodness of fit.From Table 2,it can be seen that the Freundlich adsorption model was more suitable than the Langmuir model because of the higher correlation coefficients.Also,the n values of the Freundlich model for Cd2+ions were calculated in the range of 1–10(Table 2),suggesting that the adsorption was favorable.In addition,the 1/n value was found to be less than unity(0.381),which suggests a chemisorption process[18].
| 图表编号 | XD0032559300 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.01 |
| 作者 | 李青翠、韦美彩、黄以军 |
| 绘制单位 | 河池学院化学与生物工程学院、河池学院化学与生物工程学院、河池学院化学与生物工程学院 |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 2 Adsorption isotherm parameters for Cd2+adsorption on MCM-41”的人还看了
-
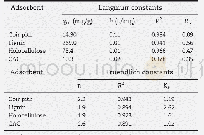
- Table 3–Langmuir and Freundlich adsorption isotherm for TMA adsorption by coir pith, lignin and holocellulose extracted