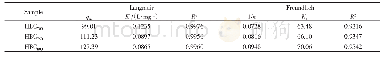
《Table 3.Fitting parameters for As adsorption by Freundlich and Langmuir isotherm models》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"Synthesis, characterization, and property test of crystalline polyferric sulfate adsorbent used in treatment of contaminated water with a high As(Ⅲ) content"》
where Q is the amount of As adsorbed on the samples at equilibrium;C is the As equilibrium concentration in solution(μg/m L);and K and 1/n are the Freundlich constants.K represents the adsorption of As at equilibrium concentrations,and 1/n indicates the degree to which adsorption is a function of As concentration.The results of these parameters obtained by nonlinear fitting are summarized in Table 3.According to the results,the K value in P-1.0 is the maximum indicating the largest adsorption capacity.The 1/n values for all the three samples are less than unity,suggesting a greater completion for adsorption sites,which become limited as the solute concentration in solution increases[27].The R2 obtained from the Freundlich model fitting on the samples are 0.85,0.52,and 0.92,indicating a poor degree of fitting on P-1.0 and better on P-0.8 and P-1.2,which could be attributed to a difference in the inner structure of the samples.
| 图表编号 | XD002881200 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.10.01 |
| 作者 | Ping-chao Ke、Zhi-hong Liu、Lin Li |
| 绘制单位 | School of Metallurgy and Environment, Central South University、School of Metallurgy and Environment, Central South University、The Robert M.Buchan Department of Mining, Queen's University |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 3.Fitting parameters for As adsorption by Freundlich and Langmuir isotherm models”的人还看了
-
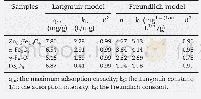
- Table 2–Parameters of Langmuir and Freundlich fits for As (V) adsorption on Zn0.6Fe2.4O4, α-Fe2O3, γ-Fe2O3, and Fe3O4.
-
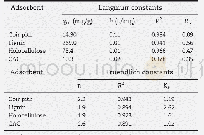
- Table 3–Langmuir and Freundlich adsorption isotherm for TMA adsorption by coir pith, lignin and holocellulose extracted