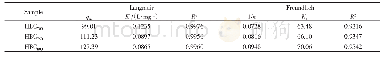
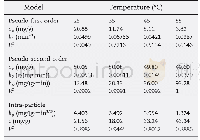
《Table 2 Kinetic modeling parameters for the adsorption of Cu (II) and Ni (II) by AC》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Adsorption of Cu and Ni Ions from Aqueous Solutions by Commercial Activated Carbon and the Reutilization in Glass Coloration》
where qe and qt are the amount of analyte(mg/g)at equilibrium and at any time,respectively.K1 and K2 are the equilibrium rate constants of pseudo-first-order and pseudo-second-order adsorption(min-1),respectively.A comparison of the R2 values(Table 2)shows that the pseudo-second-order kinetic model fits better with the higher values data(R2=0.999 6 and 0.999 5)for both Cu(II)and Ni(II).The results suggest that the adsorption of Cu(II)and Ni(II)ions on the AC adsorbent is controlled by the chemical process through the sharing of electrons or covalent forces through exchanging of electrons between adsorbent and adsorbate[21].
| 图表编号 | XD0043324800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.01 |
| 作者 | 黄美苓、WANG Chao、刘世权 |
| 绘制单位 | Department of Materials Science and Engineering, University of Jinan、Department of Materials Science and Engineering, University of Jinan、Department of Materials Science and Engineering, University of Jinan |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 2 Kinetic modeling parameters for the adsorption of Cu (II) and Ni (II) by AC”的人还看了
-
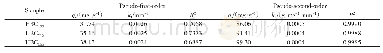
- Table 4 Adsorption kinetics parameters for Pseudo-first-order model and Pseudosecond-order model of Ni2+on HBCx