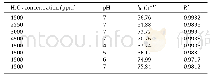
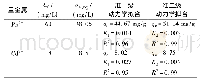
《Table 3–Pseudo-second order and pseudo-first order kinetic parameters of the adsorption of MB on Co
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Effect of Co(Ⅱ) dopant on the removal of Methylene Blue by a dense copper terephthalate》
The adsorption kinetics of Co22-CuBDC and CuBDC were studied at 30°C using MB solution at initial concentration of100 mg/L.The MB adsorption of Co22-CuBDC and CuBDC were fitted by using pseudo-first order and pseudo-second order models as shown in Fig.12.The data were best fitted with pseudo-second order models(R2>0.99).As the adsorption kinetics of these two materials fits well with the pseudosecond order model,this suggests that the adsorption process of MB may influence by chemisorption.The pseudo-second order rate constant(k2)and the amount of MB adsorbed at equilibrium(Qe,cal)for each material were calculated from the slope and the intercept and were presented in Table 3.The observed and calculated adsorption capacities are in good agreement where Co22-CuBDC shows a minor drop of adsorption performance.This could happen since doping of Co(II)can be done in such a large range of metal content,e.g.,up to 22%.Introducing such amount of Co(II)might exceed the optimal dopant content that yields the highest adsorption capacity(Chekuri and Tirukkovalluri,2017).To prove this assumption,furtherinvestigationontheadsorption performance of CuBDC containing different amounts of Co(II)need to be conducted.
| 图表编号 | XD0033528800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.07.15 |
| 作者 | Chompoonoot Nanthamathee |
| 绘制单位 | Division of Chemistry, Department of Science, Walailak University |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 3–Pseudo-second order and pseudo-first order kinetic parameters of the adsorption of MB on Co22-CuBDC and CuBDC at”的人还看了
-
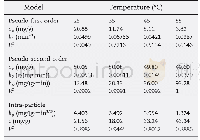
- Table 4 Adsorption kinetics parameters for Pseudo-first-order model and Pseudosecond-order model of Ni2+on HBCx
-
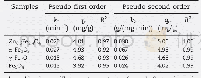
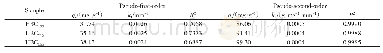
- 表4 Fe-SSBC吸附Cd2+和Pb2+的准一级和准二级参数Stable 4 Kinetic parameters of pseudo-first order and pseudo-second order of Fe-SSBC for