《Table 3 Pseudo-first-and second-order parameters for the adsorption》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Functionally Modified Cellulose Nanocrystals as an Adsorbent for Anionic Dyes》
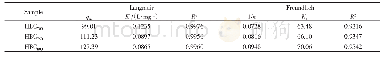
The adsorption kinetics of CNC-PDMAEMA based on the pseudo first-and second-order models are shown in Fig.7,and the estimated kinetic parameters are listed in Table 3.At the tested temperatures,the R2 for the second-order model was>0.99,while that for the firstorder model was found to be within the range 0.84~0.88.Moreover,the Qe,cal values(81.56 mg/g at 20℃,89.58mg/g at 30℃)for the pseudofirst-order model were much lower than the Qe,exp values(99.69 mg/g at 20℃,114.80mg/g at 30℃).On the other hand,the Qe,cal values(111.11mg/g at 20℃,125.00 mg/g at30℃)for the second-order model matched well with the Qe,exp values.This indicates that the adsorption kinetics of CNC-PDMAEMA were better described by the pseudosecond-order model.Thissuggests that the adsorption of the anionic dye onto the CNC-based adsorbent was mainly controlled by chemisorption[25-26].The pseudo-second-order kinetics of dye adsorption have also been reported for other biomass-based materials[25,27].
| 图表编号 | XD0020754400 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.10.01 |
| 作者 | Ming Li、ShiYu Fu |
| 绘制单位 | State Key Laboratory of Pulp and Paper Engineering, South China University of Technology、State Key Laboratory of Pulp and Paper Engineering, South China University of Technology |
| 更多格式 | 高清、无水印(增值服务) |