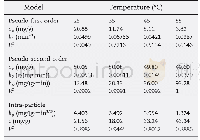
《Table 4 Estimated kinetic parameters of the two adsorption isotherms》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Functionally Modified Cellulose Nanocrystals as an Adsorbent for Anionic Dyes》
The adsorption isotherms are shown in Fig.8(b)and Fig.8(c)and the model parameters are summarized in Table 4.As we can see,the Langmuir isotherm model fitted the experimental data well(high correlation coefficients,R2=0.99).The theoretical maximum adsorption capacity(Qe,cal=136.43 mg/g at 20℃,Qe,cal=162.87 mg/g at 30℃)matched well with the experimental values(Qe,exp=123.95 mg/g at 20℃,Qe,exp=152.93 mg/g at 30℃).Therefore,it can be stated that the adsorption of AO7 onto CNC-PDMAEMA showed a monolayer uniform adsorption behavior[28-29].Moreover,the Freundlich isotherm model also described the adsorption accurately.The values of n were in the range of 1~10,indicating a favorable adsorption process[4,13,30].
| 图表编号 | XD0020754500 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.10.01 |
| 作者 | Ming Li、ShiYu Fu |
| 绘制单位 | State Key Laboratory of Pulp and Paper Engineering, South China University of Technology、State Key Laboratory of Pulp and Paper Engineering, South China University of Technology |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 4 Estimated kinetic parameters of the two adsorption isotherms”的人还看了
-
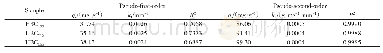
- Table 4 Adsorption kinetics parameters for Pseudo-first-order model and Pseudosecond-order model of Ni2+on HBCx