《Table 3 Adsorption kinetics parameters》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《低成本制备MCM-41介孔分子筛及其对镉离子的吸附性能(英文)》
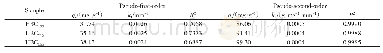
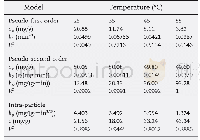
For the purpose of this kinetic study,metal ion solutions with an initial concentration of 25 mg/L were agitated in the presence of 130 mg of adsorbent at 25°C for different contact times(10,20,30,45,60,75,and120 min).The kinetic experimental data were fitted to the pseudo-first-order and pseudo-second-order kinetic models as shown in Fig.11a and Fig.11b,respectively.The pseudo-first-order kinetic model had poor convergence against the experimental data,suggested by a correlation coefficient of 0.93.However,the pseudo-second-order rate equation for the adsorption of Cd2+ions by MCM-41 converged well to theexperimental data,with a straight line fit and correlation coefficient of 0.98.Table 3 lists the absorption kinetic parameters.
| 图表编号 | XD0032559100 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.01 |
| 作者 | 李青翠、韦美彩、黄以军 |
| 绘制单位 | 河池学院化学与生物工程学院、河池学院化学与生物工程学院、河池学院化学与生物工程学院 |
| 更多格式 | 高清、无水印(增值服务) |