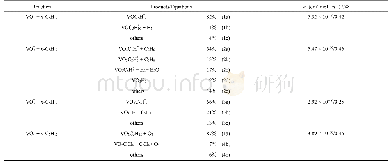
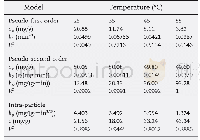
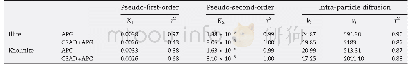
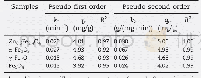
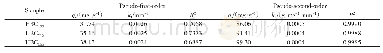
《Table 3–Parameters of pseudo-first-order and pseudo-second-order fits for As (V) adsorption on Zn0.
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Enhanced adsorption of arsenate by spinel zinc ferrite nano particles: Effect of zinc content and site occupation》
k1and k2:the equilibrium rate constants of pseudo-first-order and pseudo-second-order kinetics,respectively.
The p H dependence of Zn and Fe release from Zn0.6Fe2.4O4in aqueous solution was investigated,and the results are shown in Appendix A Fig.S2.The amounts of released Zn and Fe were quite small at pH=3–11,suggesting the high stability of Zn0.6Fe2.4O4over a wide pH range.Considering that natural water in the environment is close to neutral,the following work was carried out at the adsorption pH of 7.0.Adsorption isotherms of Zn0.6Fe2.4O4compared with other iron oxides are shown in Fig.5a.Zn0.6Fe2.4O4exhibited much higher adsorption capacity towards As(V)than the iron oxides,includingα-Fe2O3,γ-Fe2O3,and Fe3O4(25°C,pH=7.0±0.1).The equilibrium adsorption data were further fitted by the Langmuir and Freundlich models.The corresponding parameters are listed in Table 2.For all four adsorbents,the correlation coefficients(R2)of the Langmuir model were higher than for the Freundlich model.Therefore,the Langmuir model was more suitable than the Freundlich model to describe the adsorption process.The theoretical maximum adsorption capacities(Zhuang et al.,2016)of the four adsorbents calculated from the Langmuir model followed the order Zn0.6Fe2.4O4>α-Fe2O3>Fe3O4>γ-Fe2O3.Moreover,the adsorption capacity of Zn0.6Fe2.4O4was higher than most of the iron-based adsorbents in the literature at low arsenate concentrations(Appendix A Table S1).
| 图表编号 | XD0052060600 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.05.15 |
| 作者 | Can Wu、Yunyun Xu、Si Xu、Jingwei Tu、Chen Tian、Zhang Lin |
| 绘制单位 | School of Environment and Energy, The Key Laboratory of Pollution Control and Ecosystem Restoration in Industry Clusters (Ministry of Education), South China University of Technology、Guangdong Engineering and Technology Research Center for Environmental N |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 3–Parameters of pseudo-first-order and pseudo-second-order fits for As (V) adsorption on Zn0.6Fe2.4O4, α-Fe2O3, γ-”的人还看了
-
- Table 4 Adsorption kinetics parameters for Pseudo-first-order model and Pseudosecond-order model of Ni2+on HBCx
-
- Table 3 Co-occurrence model average estimates of occupancy(psi)and detection parameters(p and r)of two sympatric Gallifo
-

- 表2 Cu O-NPs吸附As (III) 的动力学参数值Tab.2 Kinetics parameters of the pseudo-first-order model and the pseudo-second-order model