《Table 1 Products, pseudo-first-order rate coefficients (k) , and reaction efficiencies (Φ) for the
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"VO_(1-4)~+阳离子与n-C_mH_(2m+2)(m=3,5,7)烷烃的反应性研究:氧含量和碳链长度的影响(英文)"》
aThe reactant-gas-pressure dependent experiments are shown in Fig.S2.
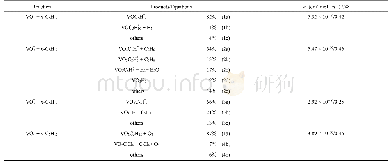
The VO+1-4cationic were generated,mass-selected,confined,and cooled(Fig.S1a,e,i,m,Supporting Information),and then they interacted with linear alkanes C3H8,n-C5H12 and n-C7H16in the ion trap reactor(Fig.S1).Among the investigated reactions,the reactivity of VO2+is higher than other three cations.For VO2+,after the interactions of VO2+with 12 m Pa C3H8 for about 0.91 ms as shown in Fig.S1f,product peaks assigned as VO2C3H8+and VO2C3H6+(Equations S3a,b in Table S1)were generated,the adoption channel and dehydrogenation channel are the major channel.Upon the interaction of VO2+with 3.6 m Pa n-C5H12 for about 1.3 ms in the reactor as shown in Fig.1d,the channels of C―C and C―H bonds activation of n-C5H12 with generation of VO2C3H6+(Equation 2a in Table 1),VO2C2H6+(Equation 2b in Table 1),and VOC5H8+(Equation 2c in Table 1)are much more obvious;some weak peaks,assigned to VO2H2+,VO2C5H8+,VO2C5H+10and VO2C5H+12(Equations 2d,e in Table 1),were also observed.Upon the reactions of VO2+with 1.3 m Pa n-C7H16 for 1.01 ms(Fig.S1h),the channels of C―C bond activation of n-C7H16 leading to VO2Cn H+2n(n=3,4,5)(Equations S4a–c in Table S1) and lighter alkanes are obvious.It can be seen that C―C bond activation of n-C5H12mediated by VO2+become the major channel as the carbon chain length increasing.Moreover,oxygen atom transfers product C2H6O shows up in the reaction of VO2+and n-C7H16.
| 图表编号 | XD0058858200 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.05.15 |
| 作者 | 赵越、崔佳桐、胡继闯、马嘉璧 |
| 绘制单位 | 北京理工大学化学与化工学院团簇科学重点实验室、北京理工大学化学与化工学院团簇科学重点实验室、北京理工大学化学与化工学院团簇科学重点实验室、上海卫星装备研究所上海裕达实业有限公司、北京理工大学化学与化工学院团簇科学重点实验室 |
| 更多格式 | 高清、无水印(增值服务) |





