《Table 3 Pseudo-second-order kinetic parameters obtained at different temperatures》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"Study of Isothermal Equilibrium,Kinetics and Thermodynamics of Adsorptive Desulfurization on Synthesized Cu~ⅠY~ⅢY Zeolite"》
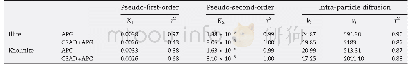
In order to thoroughly investigate the influence of temperature on adsorption capacity,the adsorption experiments run at 303 K,313 K,and 323 K,respectively,in5,10,15,20,25,30,40,50,and 60 min were studied.Figure 9 shows that t/qt decreases with an increasing temperature,confirming that the adsorption capacity qt increases gradually.To gain more insight into the reality,the experimental data were fitted in with the pseudo-second-order model,with the correlation parameters listed in Table 3.As shown by Table 3,the theoretical adsorptive amount of BT over the CuIYIIIY zeolite(qe)and the pseudo-second-order rate constant(k2)are both associated with the temperature and would rise with an increase in temperature.Overall,the adsorption of BT over the CuIYIIIY zeolite is more effective at higher temperature.
| 图表编号 | XD0011299700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.06.30 |
| 作者 | Li Xiaojuan、Song Hua、Chang Youxin |
| 绘制单位 | College of Chemistry and Chemical Engineering,Northeast Petroleum University、College of Chemistry and Chemical Engineering,Northeast Petroleum University、Provincial Key Laboratory of Oil and Gas Chemical Technology,Northeast Petroleum University、Provincia |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 3 Pseudo-second-order kinetic parameters obtained at different temperatures”的人还看了
-
- Table 3–Parameters of pseudo-first-order and pseudo-second-order fits for As (V) adsorption on Zn0.6Fe2.4O4, α-Fe2O3, γ-