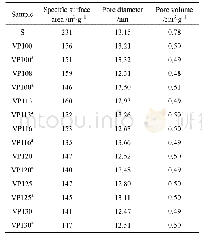
《Table 1 BET surface area of the samples》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Highly Efficient Removal of Organic Dyes by Novel As-synthesized AgBr/montmorillonite Composite》
The adsorptive and photocatalytic activities of AgBr/Mt composite were evaluated in the removal of typical dyes(RhB and MO).As displayed in Fig.6(a),compared with Na–Mt and AgBr,RhB dye in water can be efficiently removed by AgBr/Mt.After 60min,through the combined action of adsorption and photodegradation,nearly 100%of RhB was removed by AgBr/Mt composite,while,the removal efficiencies of Na–Mt and AgBr are 33%and 80%for RhB(Fig.6 (a)) ,respectively.In addition,one may note from Fig.6(a)that AgBr/Mt composite exhibits higher adsorbability for RhB than that of Na–Mt and AgBr.Generally,a larger specific surface area means more accessible adsorption sites for organic pollutants.The surface area of Na–Mt,AgBr and AgBr/Mt samples is listed in Table 1.Although the surface area of Na–Mt is higher than that of AgBr/Mt composite,Na–Mt shows lower adsorption ability than AgBr/Mt.This result indicates that the enhanced adsorption capacity of AgBr/Mt for RhB is not only due to the high surface area.Na–Mt with layer structure has permanent negative charge[26],which makes it perform well in the adsorption of cationic RhB dyes.The zero point of charge(pHzpc)for AgBr is about 5.2[27].At ranges lower or higher than the pHzpc,AgBr is positively charged or negatively charged,respectively.In the neutral condition(the pH value of RhB solution is about 7),negatively charged AgBr surface is available for the adsorption of cationic RhB.Therefore,one can deduce that AgBr/Mt composite could easily adsorb RhB molecules by electrostatic attraction.As shown in Fig.6(b),we can see that AgBr/Mt composite exhibits an excellent removal efficiency for MO compared with Na–Mt and AgBr.Notably,three samples show low adsorption capacity for MO(Fig.6 (b)) because MO is an anionic dye.Therefore,the removal of MO was mainly achieved by the photocatalytic process(Fig.6 (b)) .After 40 min exposure to visible light,10%,and 81%of MO was degraded by Na–Mt and pure AgBr,respectively.Significantly,88%of MO was decomposed over AgBr/Mt composite(Fig.6 (b)) .Obviously,the photocatalytic performance of AgBr/Mt composite for MO degradation is higher than that of Na–Mt and AgBr.
| 图表编号 | XD0043324900 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.01 |
| 作者 | 吕建昌、LI Zhenlu、HU Zheng、葛明 |
| 绘制单位 | College of Chemical Engineering, North China University of Science and Technology、College of Chemical Engineering, North China University of Science and Technology、College of Chemical Engineering, North China University of Science and Technology、College o |
| 更多格式 | 高清、无水印(增值服务) |