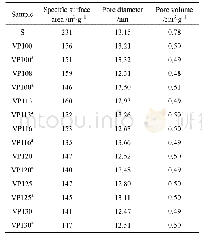
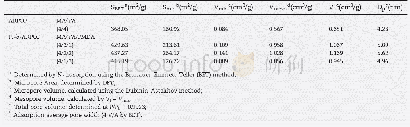
《Table 1BET specific surface area, mean pore diameter and total pore volume of the supports.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"Ce~(4+),Zr~(4+)共掺杂提高V_2O_5-WO_3/TiO_2催化剂脱硝性能及抗K中毒能力(英文)"》
The surface acidity of the catalysts was tested by NH3‐TPD,which is recognized as a key factor for the NH3‐SCR reaction[28].Fig.5 exhibits the NH3‐TPD curves of the fresh and K‐poisoning catalysts.Three desorption peaks(i.e.,I,II,and III)for the VWT catalyst appeared,resulting from the desorption of physisorbed NH3 and from the desorption of chemisorbed NH3on weak and medium‐strong acid sites,respectively[30].A new peak is detected when Ce4+and Zr4+are co‐doped,and is related to the strong acid(labeled as IV).Moreover,the amount of chemisorbed NH3 is increased significantly.There is an ob‐vious decrease after K addition,especially for the VWT and VWTZ catalysts,which is primarily owing to the combination of K with vanadium to form a V‐O‐K species that reduces surface acidity by hindering the adsorption of NH3 on Lewis or Br?nsted acid sites.Notably,the acid amount of the VWTCZ catalyst is best retained after K poisoning,and the retention is59%,which is much higher than that of other catalysts(Table2).Peng et al.[14]reported that the contact between the K species and the CeO2 surface is bonded easily to form a Ce‐O‐K structure,thereby reducing the adsorption of K on the active V species,based on the DFT study.It is well known that Zr4+can increase the thermal stability of Ce4+,which is beneficial in providing more Ce4+to combine with the K species.Therefore,the active V species can be protected better by the co‐doping of Ce4+and Zr4+.Consequently,the results of the NH3‐TPD indicate that the addition of Ce4+and Zr4+could not only increase the surface acidity of the VWT catalyst,but also effectively reduce the impact of K poisoning.
| 图表编号 | XD0028864200 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.01 |
| 作者 | 曹俊、姚小江、杨复沫、陈丽、傅敏、汤常金、董林 |
| 绘制单位 | 重庆工商大学环境与资源学院重庆市催化与环境新材料重点实验室、中国科学院重庆绿色智能技术研究院大气环境研究中心、中国科学院重庆绿色智能技术研究院大气环境研究中心、四川大学建筑与环境学院烟气脱硫国家工程研究中心、中国科学院重庆绿色智能技术研究院大气环境研究中心、重庆工商大学环境与资源学院重庆市催化与环境新材料重点实验室、南京大学现代分析中心江苏省机动车尾气控制重点实验室、南京大学现代分析中心江苏省机动车尾气控制重点实验室 |
| 更多格式 | 高清、无水印(增值服务) |