《Table 1Specific surface areas and pore volumes of Mo S2 and 3%-Ag VO3/Mo S2.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《利用AgVO_3改性MoS_2增强活性氧产生及其光催化性能的提高(英文)》
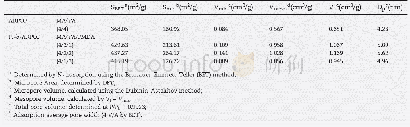
The specific surface areas and pore volumes of the prepared samples were further analyzed by nitrogen adsorption-desorption isotherm measurement.The image in the inset in Fig.4 shows that all the samples had a mesoporous pore size(approximately 25–30 nm).Furthermore,according to the IUPAC classification,both isotherms belong to type IV physisorption isotherms.The characteristic features of this type of isotherm are its hysteresis loop associated with capillary condensation occurring in the mesoporous structure,and the limiting uptake in the range of high relative pressure[36,37].The as-prepared photocatalysts displayed an H3-type hysteresis loop(P/P0>0.4),which may have resulted from the Mo S2nanoflowers consisting of several ultra-thin Mo S2 nanosheets overlapping each other.The BET surface area measurements of pure Mo S2 and 3%-Ag VO3/Mo S2 were 17.36 and 7.71 m2 g-1,respectively,demonstrating that the introduction of Ag VO3influenced the surface area.The specific surface area and pore volume calculated from the isotherms are summarized in Table1.
| 图表编号 | XD008194200 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.09.01 |
| 作者 | 秦莹莹、李红、卢健、闫永胜、逯子扬、刘馨琳 |
| 绘制单位 | 江苏大学流体机械工程与技术研究中心、江苏大学流体机械工程与技术研究中心、江苏大学绿色化学与化工技术研究院、江苏大学绿色化学与化工技术研究院、江苏大学环境与安全工程学院、江苏大学能源与动力工程学院 |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 1Specific surface areas and pore volumes of Mo S2 and 3%-Ag VO3/Mo S2.”的人还看了
-

- Table 1–Comparison of the Brunauer–Emmett–Teller (BET) surface area, pore size and pore volume of the different samples.