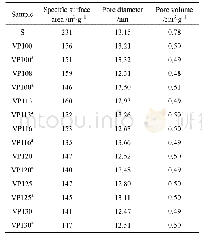
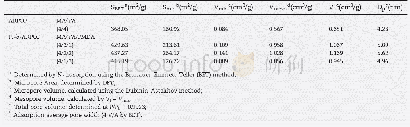
《Table 3 Specific surface areas and pore volumes of the ACs’pores》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Enhancement of gaseous mercury(Hg~0) adsorption for the modified activated carbons by surface acid oxygen function groups》
Notes:Sm:Micropore specific surface area;St:Total specific surface area;Vm:Micropore volume;Vt:Total volume
In this study,we use granular activated carbon(Calgon Carbon Co.Ltd.BPL)to prepare modified activated carbon,as shown in the parameters of Table 1.The modification method is as follows:Taking granular activated carbon,soaking them in deionized water at 100°C for 1 h;then after heating and drying,the particles are ground into size of 100-200 mesh by a sealed sample preparation pulverizer to obtain powdered activated carbon,which is recorded as BPL and then placed in a tube furnace for calcination in an inert gas atmosphere N2;the heating process is as follows:Firstly heated to 1 000°C at 10°C/min,then calcined at 1 000°C for 2 h,and finally cooled to room temperature at 10°C/min,and recorded the sample as BPL1000.Then mix 1.00 g of BPL1000 with different concentrations of benzoic acid solution,which is vibrated for 24 h at25 oC at 125 r/min,filtered to obtain activated carbon filter cake,and dried at 105°C,that is,benzoic acid modified activated carbon,referred to as BPLCXs;and then use a spectrophotometer(Thermo Electron Corporation,Genesys 10UV)to detect the concentration of residual benzoic acid in the filtrate atλ=270 nm.The calculated reduction of benzoic acid in the solution is the loading amount of benzoic acid on the surface of activated carbon.The loading amount is shown in Table 2:
| 图表编号 | XD0016467400 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.06.01 |
| 作者 | GUO Si-jia、GUO Gui-ping |
| 绘制单位 | Institute of Hydrogeology and Environmental Geology, Chinese Academy of Geological Sciences、Institute of Hydrogeology and Environmental Geology, Chinese Academy of Geological Sciences |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 3 Specific surface areas and pore volumes of the ACs’pores”的人还看了
-

- Table 1–Comparison of the Brunauer–Emmett–Teller (BET) surface area, pore size and pore volume of the different samples.