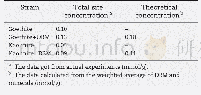
《Table 4 Content of the surface acid oxygen function groups of ACs》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Enhancement of gaseous mercury(Hg~0) adsorption for the modified activated carbons by surface acid oxygen function groups》
In this study,we use granular activated carbon(Calgon Carbon Co.Ltd.BPL)to prepare modified activated carbon,as shown in the parameters of Table 1.The modification method is as follows:Taking granular activated carbon,soaking them in deionized water at 100°C for 1 h;then after heating and drying,the particles are ground into size of 100-200 mesh by a sealed sample preparation pulverizer to obtain powdered activated carbon,which is recorded as BPL and then placed in a tube furnace for calcination in an inert gas atmosphere N2;the heating process is as follows:Firstly heated to 1 000°C at 10°C/min,then calcined at 1 000°C for 2 h,and finally cooled to room temperature at 10°C/min,and recorded the sample as BPL1000.Then mix 1.00 g of BPL1000 with different concentrations of benzoic acid solution,which is vibrated for 24 h at25 oC at 125 r/min,filtered to obtain activated carbon filter cake,and dried at 105°C,that is,benzoic acid modified activated carbon,referred to as BPLCXs;and then use a spectrophotometer(Thermo Electron Corporation,Genesys 10UV)to detect the concentration of residual benzoic acid in the filtrate atλ=270 nm.The calculated reduction of benzoic acid in the solution is the loading amount of benzoic acid on the surface of activated carbon.The loading amount is shown in Table 2:
| 图表编号 | XD0016467500 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.06.01 |
| 作者 | GUO Si-jia、GUO Gui-ping |
| 绘制单位 | Institute of Hydrogeology and Environmental Geology, Chinese Academy of Geological Sciences、Institute of Hydrogeology and Environmental Geology, Chinese Academy of Geological Sciences |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 4 Content of the surface acid oxygen function groups of ACs”的人还看了
-
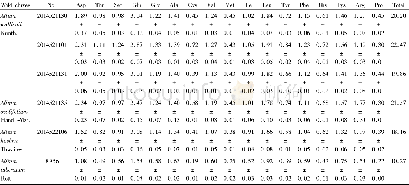
- Table 1 Content of hydrolyzed amino acids in the wild chives and cultivated chives in Hezhang County
-
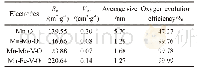
- Table 2 Specific surface area, average size, and oxygen evolution efficiency of the oxide electrodes
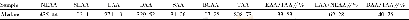


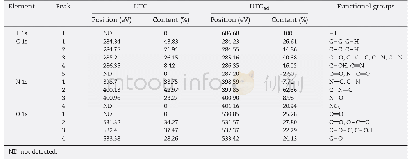
![Table 4 Functional groups of coke de posit on the spe nt catalysts[9]](http://bookimg.mtoou.info/tubiao/gif/RLHX201902005_11300.gif)