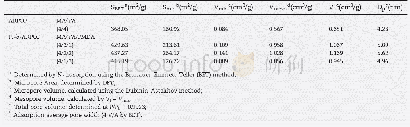
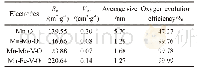
《Table 2 Specific surface area, average size, and oxygen evolution efficiency of the oxide electrode
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Oxygen Evolution Efficiency and Chlorine Evolution Efficiency for Electrocatalytic Properties of MnO_2-based Electrodes in Seawater》
Fig.6 shows the pore size distribution of the oxide electrodes.Micropores with a size between 0.85 and 2.5nm appear on the surface of Mn+Mo-O,Mn+Mo+V-O,and Mn+Fe+V-O electrodes,whereas mesopores with a size between 3.6 and 9.4 nm appear on the surface of Mn-O electrode.Relationship between average pore size and oxygen evolution efficiency of the electrodes is shown in Fig.7.Obviously,the average pore sizes of Mn+Mo-O,Mn+Mo+V-O and Mn+Fe+V-O are all no more than 2 nm with nearly 100%oxygen evolution efficiency.However,the average pore size of Mn-O is 5.7 nm with only 47.27%oxygen evolution efficiency.The specific surface area shown in Table2 was calculated from the pore size distribution.As can be seen in Table 2,the Mn+Fe+V-O electrode has the largest specific surface area of 220.64 m2·g-1and the smallest average pore size of 1.27 nm of all the oxide electrodes.The specific surface area of the Mn-O electrode is 139.55 m2·g-1,which is far less than that of Mn+Fe+V-O electrode(220.64 m2·g-1)but not much different with that of the Mn+Mo-O(146.66m2·g-1)and Mn+Mo+V-O(123.88 m2·g-1)electrodes.The Mn-O electrode has the largest average pore size of 5.7 nm.Moreover,the Mn-O electrode has the lowest oxygen evolution efficiency of 47.27%.The Mn+Mo-O,Mn+Mo+V-O and Mn+Fe+V-O electrodes have an oxygen evolution efficiency of approximately100%.This indicates that the influence of pore size is much more significant than the influence of the specific surface area on the oxygen evolution efficiency of the electrode,which reveals that the micropore structure of oxide electrodes is a positive factor in determining the oxygen evolution in simulated seawater.This may be because the small pores of the electrodes are conducive to penetration by the small hydrated ions.The primary anions in seawater are OH-and Cl-.The hydrated hydroxyl ion is smaller than the hydrated chloride ion;hence,the hydrated hydroxyl ion can penetrate into the micropores of the electrodes more easily for oxygen evolution.
| 图表编号 | XD0043325800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.01 |
| 作者 | 闫镇威、SONG Lijun、TANG Mingqi、FENG Zaiqiang |
| 绘制单位 | School of Mechanical Engineering, North China University of Water Resources and Electric Power、Environmental Protection and Chemistry Center, Suzhou Nuclear Power Research Institute、School of Materials Science and Engineering, North China University of Wa |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 2 Specific surface area, average size, and oxygen evolution efficiency of the oxide electrodes”的人还看了
-

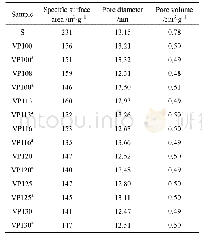
- Table 2.Specific surface area and pore volume of untreated and microwave-treated VTM with 1.18-1.7 mm particle size