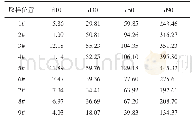
《Table 1.The particle size distribution parameters, specific surface area and pH val-ues of NCA-P1 a
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《TiP_2O_7-coated LiNi_(0.8)Co_(0.15)Al_(0.05)O_2 cathode materials with improved thermal stability and superior cycle life》
The XRD measurements were conducted to examine the crystal structure of both TiP2O7coated sample NCA-C1 and uncoated pristine sample NCA-P1,and the XRD results were shown in Fig.1(a).As can be seen,both samples can be well indexed toα-NaFeO2layered structure with a space group of rhombohedral R3m.For NCA-C1 sample,no Ti P2O7phase can be found in the XRD pattern due to the low content.The values of lattice constants a and c were respectively calculated to be 2.8673?A and 14.1916?A for NCA-P1 sample,2.8668?A and 14.1909?A for NCA-C1 sample,which indicates that there are no obvious changes in the crystal structure of NCA after coated with Ti P2O7.The c/a ratios of NCA-P1 and NCA-C1 were calculated to be 4.97 and 4.95,respectively.The rather high c/a ratios and clear splits of(018)/(110)and(006)/(012)pairs indicate that the layered structure of both as-synthesized materials were well formed[17,26].The I003/I104value of Ni-rich cathode materials was usually used to represent the degree of cation mixing between lithium ions and metal ions,and the higher I003/I104value indicated better ordered arrangement.Serious cation mixing of NCA materials was supposed to result in structural degradation during cycling[5,7,8].The I003/I104values of NCA-P1 and NCA-C1 were calculated to be 1.508 and 1.641,respectively.Since the I003/I104ratio is corresponding to the intensity of cation mixing,the higher I003/I104ratio representing lower cation mixing and better layered structure of NCA-C1,the better structural crystallinity will finally benefit the electrochemical performances of NCA-C1.The particle size distribution curves of both samples were shown in Fig.1(b),and the main tested values were exhibited in Table 1.The particle size distributions of NCA-P1 and NCA-C1 were similar with each other,the D50 value of NCA-C1 was 12.0μm,which a little higher than 11.6μm of NCA-P1 sample,which indicates that TiP2O7coating makes no obvious difference in particle size.The tested results of specific surface area and pH of NCA-P1 and NCA-C1 were also displayed in Table 1.The BET values of NCA-P1 and NCA-C1 were 0.36 and 0.43 m2/g,respectively.The larger specific surface area of NCA-C1 was mainly brought by the residual TiP2O7layer which may allow more contact area between cathode and electrolytes.The pH value is an important parameter to judge the processability of Ni-rich materials[4,27].In this work,the TiP2O7coating was supposed to consume a part of redundant lithium on the surface of NCA particles to form LixTiP2O7which favors the conductivity of Li+.The p H values were 12.04 and 11.37 for NCA-P1and NCA-C1 respectively,revealing that coating a layer of TiP2O7on the surface of NCA particles was an efficient way to decrease the alkalinity and benefit the processability.
| 图表编号 | XD0042897400 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.01 |
| 作者 | Guan Wu、Yingke Zhou |
| 绘制单位 | The State Key Laboratory of Refractories and Metallurgy, Institute of Advanced Materials and Nanotechnology, College of Materials and Metallurgy, Wuhan University of Science and Technology、The State Key Laboratory of Refractories and Metallurgy, Institute |
| 更多格式 | 高清、无水印(增值服务) |