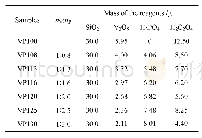
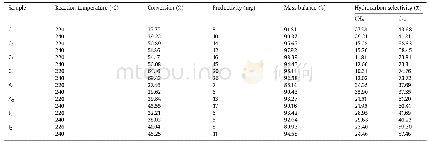
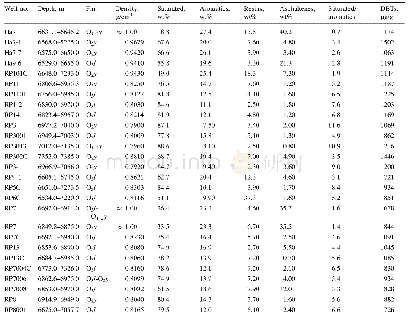
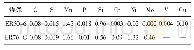
《Table 1 Compositions for preparing the oxide electrodes》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Oxygen Evolution Efficiency and Chlorine Evolution Efficiency for Electrocatalytic Properties of MnO_2-based Electrodes in Seawater》
Thermal decomposition was adopted to prepare an IrO2 coating as an intermediate layer on the titanium substrate[10,15].The IrO2 coating was prepared by the following procedure:first,a precursor of a 0.2 M chloroiridic acid butanol solution was prepared;it was then painted with a brush on the titanium substrates using three coats,which after each coat were dried at100℃for 10 min and then sintered at 450℃for 10min;then,this specimen was finally sintered for 1 h at450℃;the electrodes were then anodically polarized at 1 000 A·m-2 for 10 min in 0.1 M H2SO4 at room temperature to remove any contaminants;finally,Mn,Mn+Mo,Mn+Mo+V,and Mn+Fe+V oxide electrode coatings were prepared on the electrodes by anodic deposition at 90℃with a constant current density of 600 A·m-2 for 60 min.The composition of the resultant oxide electrodes are listed in Table 1.The pH value of the electrolyte was buffered to 0.5 by using concentrated sulfuric acid.Pure(>99.98%)titanium was employed as a counter electrode.
| 图表编号 | XD0043325700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.01 |
| 作者 | 闫镇威、SONG Lijun、TANG Mingqi、FENG Zaiqiang |
| 绘制单位 | School of Mechanical Engineering, North China University of Water Resources and Electric Power、Environmental Protection and Chemistry Center, Suzhou Nuclear Power Research Institute、School of Materials Science and Engineering, North China University of Wa |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 1 Compositions for preparing the oxide electrodes”的人还看了
-
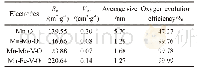
- Table 2 Specific surface area, average size, and oxygen evolution efficiency of the oxide electrodes