《Table 2 Results of X-ray photoelectron spectra for the as-prepared catalysts》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Phosphorous-Modified Carbon Nanotube-Supported Pt Nanoparticles for Propane Dehydrogenation Reaction》
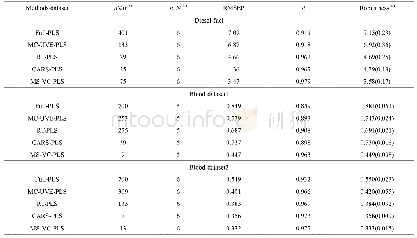
The amount of coke deposits was analysed via a TG-MS method.Shi,et al.[36]thought that the combustion of coke occurring below 400oC was attributed to the coke covering the active metal sites,while that burnt above400oC was assigned to the coke located on the surface of the support.It can be seen from Figure 5 that the amoun of coke deposits over the Pt/P-CNTs catalyst(0.4%)is remarkably smaller than that over Pt/CNTs(4.1%,Table 1)which is consistent with the results of Figures 1,2,and 4.To verify the SMSIs for Pt/P-CNTs,the MedeA-VASP software was employed to calculate the absorption energy of Pt nanoparticles over P-CNTs and CNTs supports,respectively,with the results exhibited in Figure 6 and Table1.The absorption energy of Pt over Pt/P-CNTs is higher than that over Pt/CNTs,which is in agreement with the report made by Tong,et al.[19],indicating that Pt nanoparticles can interact strongly with the P-CNTs support.The binding energy of the Pt 4f electrons for Pt/P-CNTs shifts to a higher value as compared to that for Pt/CNTs(Figure7),which is in good agreement with the literature information[28].In addition,the devolution results of Pt 4f in Figure 7 and Table 2 show that the electrons of Pt nanoparticles on P-CNTs are more electrophobic than those of Pt nanoparticles on CNTs,which may be derived from the SMSIs between Pt and P-containing groups[37].The XPS results may imply that the P doping has an effect on the electronic structure of Pt to enhance the electrical conductivity of the catalyst,which can lead to the SMSIs or even exert an influence on the catalytic performance.Additionally,Tong and colleagues considered that the SMSIs in Pt/P-CNTs might be derived from the hybridization between the hybrid orbitals of Pt and P[19].
| 图表编号 | XD0029185400 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.03.30 |
| 作者 | Liu Jie、Liu Changcheng、Da Zhijian、Zheng Huidong |
| 绘制单位 | College of Chemical Engineering, Fuzhou University、SINOPEC Research Institute of Petroleum Processing、SINOPEC Research Institute of Petroleum Processing、SINOPEC Research Institute of Petroleum Processing、College of Chemical Engineering, Fuzhou University |
| 更多格式 | 高清、无水印(增值服务) |