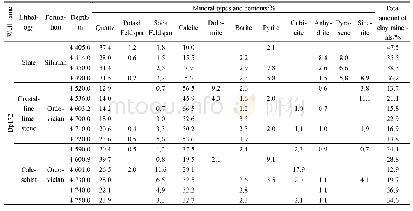
《Table 1–Extended X-ray absorption fine structure (EXAFS) results during the oxidation reaction of S
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Oxidation of antimony(Ⅲ) in soil by manganese(Ⅳ) oxide using X-ray absorption fine structure》
The EXAFS spectra and fitting results for Sb in andosol during the oxidation reaction are shown in Fig.3 and Table 1,respectively.The first measurement(RT=0 day)was clearly different from the second measurement(RT=14 day),as shown by the small peaks of the first Sb\\O bond from the EXAFS Fourier transforms at approximately 1.6?before correcting the phase shift.The coordination number was close to 3(Table 1).However,the second measurement(RT=14 day)showed the larger peaks of the first-shell Sb\\O bond and the coordination number was much larger,i.e.,closer to 6.Both the first-shell Sb\\O bonds and the distances of the two measurements were nearly the same(~1.99?).The shell Sb\\Sb bond showed a distance of 3.6?for the first measurement(RT=0 day);however,it disappeared after two weeks of the Sb(III)oxidation reaction.This indicated that the Sb2O3compounds were completely converted into other forms and possibly adsorbed by andosol.These results again confirm that Sb(III)was oxidized to Sb(V)during the two weeks of reaction and demonstrate that the oxidation states of Sb can also be discriminated based on the coordination numbers of the firstshell Sb\\O bond.Craw et al.(2004),Mitsunobu et al.(2006),and Asaoka et al.(2012)found Sb\\Fe bonds in the results reported for Sb(V)adsorbed onto ferrihydrite or iron(III)hydroxide in field soil and/or sediment samples,and Sb(III)/Sb(V)was adsorbed onto goethite or hydrous ferric oxide during adsorption and/or coprecipitation experiments(Scheinost et al.,2006;Fan et al.,2014;Guo et al.,2014b).No Sb\\Fe bonds were found in this study,possibly because we manually added Sb2O3directly to andosol,and the duration of the oxidation reaction was only two weeks.The adsorption of Sb onto iron(III)hydroxide might require more time in the soil environment.The possible reactions can be expressed as follows:
| 图表编号 | XD0025355000 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.11.15 |
| 作者 | Lei Fu、Katsumi Shozugawa、Motoyuki Matsuo |
| 绘制单位 | Graduate School of Arts and Sciences,The University of Tokyo、Graduate School of Arts and Sciences,The University of Tokyo、Graduate School of Arts and Sciences,The University of Tokyo |
| 更多格式 | 高清、无水印(增值服务) |
![Table 1 Fine varieties of Chinese milk vetch in China[3, 5-8]](http://bookimg.mtoou.info/tubiao/gif/AGBT201804008_13200.gif)