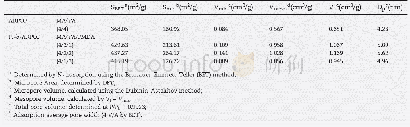
《Table 4 Specific area, pore volume and pore diameter distribution of MnFeOx nanorods》
With the development of low temperature SCR catalyst,complex transition metal oxidation catalyst has been throughly studied.Among these catalysts,the composite Mn-based catalysts were of great importance because of its wide reaction temperature range and higher denitrification efficiency.The composite Mn-basedcatalystsandtheirdenitrification performance were listed in Table 3.Li et al.[15]prepared MnFeOx nanorods used a hydrothermal method and their NOx conversion rates at different temperatures were evaluated,the results indicated that the MnFe0.1Ox nanorods exhibited the highest NO conversion rate in NH3-SCR reaction.The SCR activities of MnFeOx nanorods with different Fe/Mn molar ratios were presented,the pure Mn O2 nanorods and traditional V2O5-WO3/TiO2 catalyst were also investigated for comparison.It was found that SCR activity of V2O5-WO3/TiO2 catalyst was only less than 20%below150℃.Moreover,pure MnO2 nanorod samples obtained lower NO removal rate in the studied temperature window.It was obvious that the NO conversion rate of MnFe0.1Ox nanorods could reach approximately 100%at above 200℃,which was the best Fe/Mn molar ratio.It was reported that oxidation activity of NO to NO2 over catalysts played a key role in faster reaction:NO+NH3+1/4O2?N2+3/2H2O.Hence,the conversion of NO to NO2 in absence of NH3 on sample was researched[Fig.3(b)].It can be seen that MnFe0.1Ox nanorod catalysts obtained higher oxidation activity of NO to NO2.It was reported that NH4 ions reacted with gaseous NO2 molecules to generate NH4NO2,which was decomposed to N2 and H2O in SCR reaction.Therefore,it was illustrated that a high oxidation capacity of NO to NO2 or high NO2 content resulting in a good SCR catalytic activity as shown in Fig.3.The BET surface area of MnFeOx bimetallic oxides catalysts was larger than that of pure MnO2 nanorods and the BET surface area of the MnFe0.1Ox nanorod was larger than those of others MnFeOx bimetallic oxides catalysts as listed in Table 4.Li et al.[15]proposed the reaction mechanism of SCR reaction to remove NOx by NH3.It was supposed that a redox reaction may occur between Mn and Fe ions resulting in electronic transfer.The electronic transfer played a key role in the oxidation of NO to NO2 during the NH3-SCR reaction of removing NOx as shown in Fig.4.
| 图表编号 | XD0033431000 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.04.01 |
| 作者 | 宁汝亮、刘霄龙、朱廷钰 |
| 绘制单位 | 贵州大学化学化工学院、中国科学院过程工程研究所湿法冶金清洁生产技术国家工程实验室、中国科学院过程工程研究所湿法冶金清洁生产技术国家工程实验室、贵州大学化学化工学院、中国科学院过程工程研究所湿法冶金清洁生产技术国家工程实验室、中国科学院城市环境研究所区域大气环境卓越中心 |
| 更多格式 | 高清、无水印(增值服务) |
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式