《Table 3 The parameters of 15%MWCNTs/Cr-TiO2 isothermal adsorption model fitting》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Influence of Cr~(3+) Concentration on SO_2 Removal over TiO_2 Based Multi-walled Carbon Nanotubes》
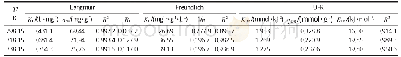
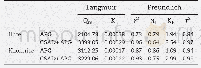
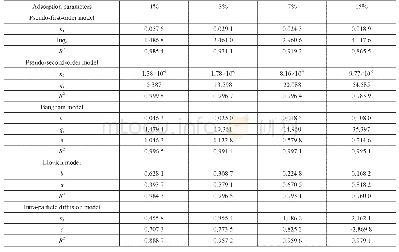
where KF(mg· (m3)1/n) /(g·g1/n)is the Freundlich constant,and1/n,between 0 and 1,reflects the adsorption intensity or surface heterogeneity.The adsorption isotherm can provide further information on the adsorption behavior of an adsorbent Figure 8 shows the adsorption isotherms of adsorbents a different temperatures.Table 3 lists the parameters,which were obtained by fitting the experimental data with both models.As shown in Table 3,the R2 values of the Langmuir and Freundlich models were higher than 0.98,suggesting that both can predict the SO2 adsorption equilibrium on the sample surface and that the adsorption process of the sample was dominated by chemical adsorption.As the temperature increased,qm decreased.Thus,an increased temperature was not conducive to the reaction.
| 图表编号 | XD0029185700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.03.30 |
| 作者 | Yang Yanchun、Jia Fengrui、Jian Weiwei、Zhang Shaopeng、Ma Danzhu、Liu Guangxin |
| 绘制单位 | College of Petroleum Engineering, Liaoning Shihua University、College of Petroleum Engineering, Liaoning Shihua University、College of Petroleum Engineering, Liaoning Shihua University、College of Petroleum Engineering, Liaoning Shihua University、College of |
| 更多格式 | 高清、无水印(增值服务) |