《Table 4 Thermodynamic parameters of adsorption process at different fix-bed temperatures》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Influence of Cr~(3+) Concentration on SO_2 Removal over TiO_2 Based Multi-walled Carbon Nanotubes》
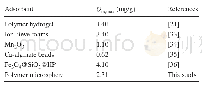
As shown in Fig.9,low temperature favored the progress of SO2 adsorption.The most favorable adsorption temperature was 333.15 K.The thermodynamic adsorption parameters of the sample for SO2 can be calculated based on the fitting results,as shown in Table4.?H was-43.64 kJ/mol,and?S was–21.06 J/(mol·K).As shown in Table 4,the free energy of adsorption,?G,became negative,indicating that the adsorption of SO2by the sample was a spontaneous process.In addition,as the temperature increased,the absolute value of?G decreased,indicating that with the increase of adsorption temperature,the spontaneous tendency of the adsorption process decreased,suggesting that a temperature increase was not conducive to adsorption process.The change in adsorption enthalpy was negative,indicating that the adsorption process was an exothermic reaction.The negative change in entropy(?S)reflected the decreased randomness on the adsorbent during the adsorption process.The decrease in the degree of freedom of the adsorbate molecules was not conducive to adsorption.
| 图表编号 | XD0029185800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.03.30 |
| 作者 | Yang Yanchun、Jia Fengrui、Jian Weiwei、Zhang Shaopeng、Ma Danzhu、Liu Guangxin |
| 绘制单位 | College of Petroleum Engineering, Liaoning Shihua University、College of Petroleum Engineering, Liaoning Shihua University、College of Petroleum Engineering, Liaoning Shihua University、College of Petroleum Engineering, Liaoning Shihua University、College of |
| 更多格式 | 高清、无水印(增值服务) |