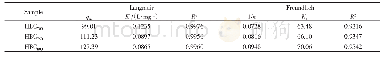
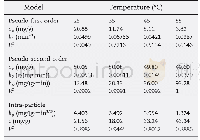
《Table 2 Kinetic models constants for Li+adsorption on adsorbents》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Highly Selective Lithium Ion Adsorbents:Polymeric Porous Microsphere with Crown Ether Groups》
where Q m(mg/g)is the maximum adsorptive capacity;K L(L/mg)and K F( (mg/g)(L/mg) 1/n) represent the Langmuir and Freundlich constants,respectively;and n is the heterogeneity factor.Figure 7 b,c show the data fi tted with the Langmuir and Freundlich isotherm models,respectively,and Table 1 lists the related model parameters and correlation coeffi cients.The correlation coeffi cient(R 2)for the Langmuir model(0.9986)is much higher than that for the Freundlich model(0.9102),which means that the Langmuir model is more eff ective in describing the adsorption process.This result indicates that the adsorption sites are basically homogenous.
| 图表编号 | XD0076870900 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.04.01 |
| 作者 | Caideng Yuan、Lei Zhang、Haichao Li、Ruiwei Guo、Meng Zhao、Lan Yang |
| 绘制单位 | School of Chemical Engineering and Technology,Tianjin University、School of Chemical Engineering and Technology,Tianjin University、School of Chemistry and Chemical Engineering,Qinghai Nationalities University、School of Chemical Engineering and Technology,T |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 2 Kinetic models constants for Li+adsorption on adsorbents”的人还看了
-

- 表2 CPDB-MONT和Al-CPDB-MONT吸附Cr (Ⅵ) 的动力学参数Tab.2 Kinetic parameters for Cr (Ⅵ) adsorption on CPDB-MONT and Al-CPDB-MONT