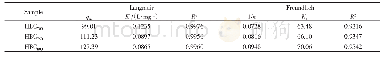
《Table 2–Parameters of Langmuir and Freundlich fits for As (V) adsorption on Zn0.6Fe2.4O4, α-Fe2O3,
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Enhanced adsorption of arsenate by spinel zinc ferrite nano particles: Effect of zinc content and site occupation》
The crystal structures of the as-synthesized ZnxFe3-xO4samples with different zinc contents were determined by XRD(Fig.1).The diffraction patterns confirmed the formation of the pure spinel phase for all five samples.The diffraction peaks at 2θ=18.28°,30.12°,35.39°,42.90°,53.38°,56.99°,and62.41°corresponded to the(111),(220) ,(311) ,(400) ,(422) ,(511) ,and(440)planes of ZnFe2O4(JCPDS:001-1109),respectively,indicating the successful synthesis of ZnxFe3-xO4with different zinc contents.With as the x value increased,a small peak appeared at 2θ=31.70°,which belonged to the(001)plane of ZnO(JCPDS:005–0664),suggesting a small amount of ZnO present in the ZnxFe3-xO4product with increasing Zn dosage.The valence state of iron in the sample was determined by XPS.The high-resolution XPS spectra of Fe 2p(Appendix A Fig.S1)showed a satellite peak at 719 eV between the two characteristic peaks of Fe 2p 3/2 and Fe 2p 1/2.This peak was the fingerprint of the electronic structure of Fe3+,which also indicated the absence of Fe2+(Tian et al.,2015).It was reported that in the XRD pattern of spinel ferrite,the intensity of the(220)peak reflects the occupation of metal cations in Tdsites,while the intensity of the(222)peak reflects the occupation of metal cations in Ohsites(Slovaca,1997).Therefore,a higher(220)to(222)peak intensity ratio(I (220)/I(222)) indicated that more zinc atoms occupied the Tdsite,while lower I(220)/I(222)indicated that more zinc atoms occupied the Ohsite.The I(220)/I(222)values calculated from the XRD patterns of ZnxFe3-xO4are shown in Table 1.It can be seen that the I(220)/I(222)value changed slightly with increasing x from 0.2 to 0.6.However when x>0.6,the I(220)/I(222)ratio increased markedly with increasing x.This result indicated that the zinc atoms mainly occupy the octahedral sites when x<0.6 and transfer to the tetrahedral sites gradually when x>0.6.
| 图表编号 | XD0052060300 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.05.15 |
| 作者 | Can Wu、Yunyun Xu、Si Xu、Jingwei Tu、Chen Tian、Zhang Lin |
| 绘制单位 | School of Environment and Energy, The Key Laboratory of Pollution Control and Ecosystem Restoration in Industry Clusters (Ministry of Education), South China University of Technology、Guangdong Engineering and Technology Research Center for Environmental N |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 2–Parameters of Langmuir and Freundlich fits for As (V) adsorption on Zn0.6Fe2.4O4, α-Fe2O3, γ-Fe2O3, and Fe3O4.”的人还看了
-
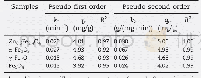
- Table 3–Parameters of pseudo-first-order and pseudo-second-order fits for As (V) adsorption on Zn0.6Fe2.4O4, α-Fe2O3, γ-
-

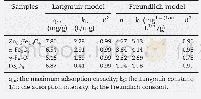
- Table 2–Langmuir and Freundlich isotherms fitting parameters for glyphosate adsorption and maximum recorded adsorbed amo