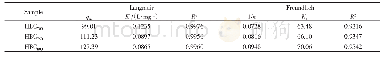
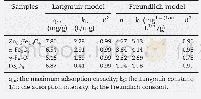
《Table 3–Isotherms for Hg (II) adsorption on S-IIPs and NIPs at different temperatures.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"Carboxymethyl cellulose thiol-imprinted polymers:Synthesis, characterization and selective Hg(Ⅱ) adsorption"》
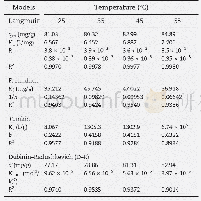
qm:theoretical maximum adsorption capacity;KL:free energy of adsorption constant;RL:dimensionless separation factor;Kf:adsorption capacity constant;1/n:adsorption intensity constant;Kt:isotherm constant;b:heat of adsorption constant;KDR:adsorption energy
Calculated isotherm constants are shown in Table 3.The correlation coefficients related to the Langmuir isotherms were much closer to 1 in comparison to the other applied adsorption isotherms for the corresponding temperatures This suggests that the adsorption of mercury was predominately a monolayer process without mutual interactions between the adsorbed molecules.Other adsorption processes describedbytheFreundlich,Temkin,andDubinin–Radushkevich isotherms are less likely to occur.A dimensionless separation factor(RL)defines the favourability of an adsorption process and is described by Eq.(20).
| 图表编号 | XD0052062600 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.05.15 |
| 作者 | Tarisai Velempini、Kriveshini Pillay、Xavier Y.MbiANDa、Omotayo A.Arotiba |
| 绘制单位 | Department of Applied Chemistry, University of Johannesburg, Doornfontein Campus、Department of Applied Chemistry, University of Johannesburg, Doornfontein Campus、Centre for Nanomaterials, University of Johannesburg, Doornfontein Campus、Department of Scien |
| 更多格式 | 高清、无水印(增值服务) |