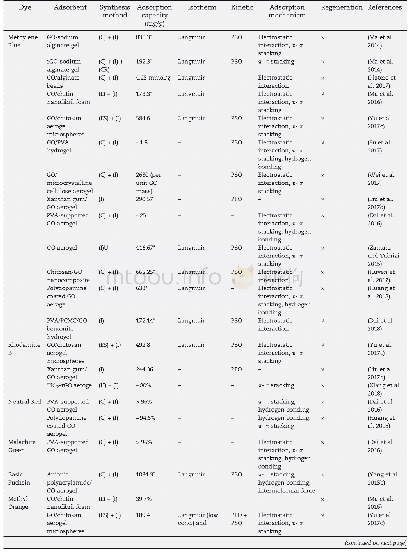
《Table 4Calculated surface adsorption energies Eads for EC and H2 adsorption on Cu (111) surface and
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《碳酸乙烯酯选择加氢合成甲醇与乙二醇高效稳定Cu/SiO_2催化剂研究(英文)》
DFT calculations showed the configurations of EC and H2molecules adsorbed on the of Cu(111)surface(Fig.9).These surface adsorption energy results are summarized in Table 4.Fig.9(a)and Fig.9(b)show the optimized geometries—the carbonyl O is closer to the Cu(111)surface than the ring O sug‐gesting that the interaction between the carbonyl O and the Cu(111)surface is the main contributor to the adsorption.However,the distances were 0.2747 and 0.3004 nm,respec‐tively,which means that the van Edward force was the main type of interaction between two atoms.That is,the EC molecule was not activated by the Cu(111).This was confirmed by com‐paring the adsorption energy of two similar configurations( (a)and(b)) :Configuration a has a weaker binding strength(Eads=–0.921 and–1.021 kcal mol–1)corresponding to a weaker O‐surface interaction.
| 图表编号 | XD008190900 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.08.01 |
| 作者 | 刘佳驹、贺鹏、王利国、刘辉、曹妍、李会泉 |
| 绘制单位 | 中国科学院过程工程研究所中国科学院绿色过程与工程重点实验室、北京化工大学、中国科学院过程工程研究所中国科学院绿色过程与工程重点实验室、中国科学院过程工程研究所中国科学院绿色过程与工程重点实验室、盐城工学院江苏省生态建材与环保装备协同创新中心、中国科学院大学中丹学院、北京化工大学、中国科学院过程工程研究所中国科学院绿色过程与工程重点实验室、中国科学院过程工程研究所中国科学院绿色过程与工程重点实验室、中国科学院大学中丹学院 |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 4Calculated surface adsorption energies Eads for EC and H2 adsorption on Cu (111) surface and Cu+/Si O2.”的人还看了
-
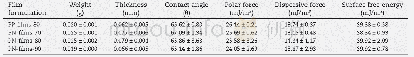
- Table 2–Weight, thickness, contact angle, polar force, dispersive force and surface free energy of PP-films and PN-films
-
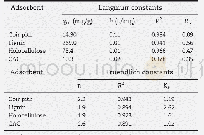
- Table 3–Langmuir and Freundlich adsorption isotherm for TMA adsorption by coir pith, lignin and holocellulose extracted