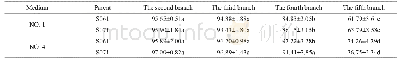
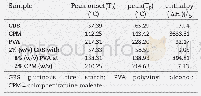
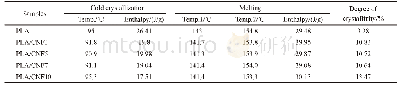
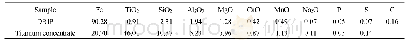《Table 1 Experimental atomic compositions obtained from the areas of core level photoemission peaks
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Preparation and Properties of Poly(ethylene terephthalate )(PET) Coated by Chitosan( CS) Based on Sol-gel Method》
XPS spectra of pristine PET and modified PET fiber are show n in Fig.2.The C1s and O1s peaks are found in pristine PET fiber w ith O/C atom ratio of 0.21(Table 1).The XPS spectra of SE-PET fiber are similar to those of pristine PET fiber,w hile the oxygen content increased(O/C atom ratio=0.37,Table 1).N1s peak can be observed,indicating the presence of CS on PET fiber.The Na1s peak can be explained by the residual of NaOH during CS gelation process.Figure 3presents the C1s core level spectra of three kinds of PET fiber.The spectra of C1s core level obtained from pristine PET fiber are show n in Fig.3(a)and can be deconvoluted into three peaks with binding energies at about 284.5 e V(C—C or C—H),286.5 e V(C—O),and 288.9 e V(O—CO),respectively.It is easy to find no new peak appears in Fig.3(b),indicating that PET and SE w ere bonded by hydrogen bonds and Van Edw ard force.M oreover,due to a large number of hydroxyl and ester groups on SE,the peaks intensity both at 286.5 eV(C—O)and288.9 eV(O—CO)are enhanced remarkably.What's more,it can be seen that a new peak at 288.2 eV emerges in Fig.3(c),w hich corresponds to N—CO bond,indicating that CS has been grafted onto SE-PET fiber successfully.
| 图表编号 | XD0014952100 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.12.31 |
| 作者 | 袁凯、胡祖明、段广宇、于俊荣、王彦、诸静 |
| 绘制单位 | College of Material Science and Engineering,Donghua University、College of Material Science and Engineering,Donghua University、State Key Laboratory for Modification of Chemical Fibers and Polymer Materials,Donghua University、State Key Laboratory for Modifi |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 1 Experimental atomic compositions obtained from the areas of core level photoemission peaks corrected by sensitiv”的人还看了
-
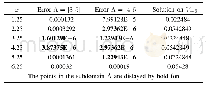
- TABLE III THE ERRORS ARE THE DIFFERENCE BETWEEN THEMULTI-SCALE RESULTS AND THE RESULTS OBTAINED USINGV-5, WITH h=0.01 AT