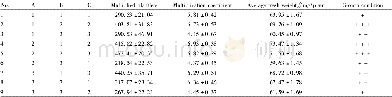
《Table 2.Obtained electrochemical parameters from EIS tests on the mild steel specimens immersed in
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Azole derivatives embedded in montmorillonite clay nanocarriers as corrosion inhibitors of mild steel》
Note:Y0,dl is the admittance of the constant phase element of double layer,Y0,f is the admittance of the constant phase element of inhibitor film,ndl is the exponent of the constant phase element of double layer,nf is the exponent of the constant phas
Fig.2 shows the Bode plots used to evaluate the corrosion protection of the mild steel samples in different solutions.In these figures,a single time constant is observed for the extracted solutions containing MBI,MBT,Na+-MMT,or MMT+MBI,whereas a double time constant is observed after 4 h for the sample immersed in an extracted solution containing MMT+MBT,indicating the generation of an inhibitor sediment layer on the metal surface.The equivalent circuits used for fitting the data of the single and double time constant systems are shown in Fig.3.The double time constant in the bode plot corresponding to steel corrosion protection in the MMT+MBT solution(Fig.2 (f)) might be due to the adsorption of the inhibitor onto steel surface.This adsorption process led to a small low-frequency loop observed as a resistance and confirmed by the data in Table 2.This resistance is parallel with a capacitance of the adsorbed film;the constant phase element(CPE)of the film is represented as CPEf in Fig.3.
| 图表编号 | XD0028409900 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.01 |
| 作者 | Milad Edraki、Davood Zaarei |
| 绘制单位 | Polymer Department, Technical Faculty, South Tehran Branch, Islamic Azad University、Polymer Department, Technical Faculty, South Tehran Branch, Islamic Azad University |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 2.Obtained electrochemical parameters from EIS tests on the mild steel specimens immersed in the extracted solutio”的人还看了
-

- 表4 Ni-Ti N纳米镀层的电化学阻抗谱拟合参数Table 4 Fitting parameters from electrochemical impedance spectra of Ni-Ti N nanocoatings
-

- Table 5 Eff ect of Mn/Mo ratio on macroscopic kinetic parameters of benzaldehyde generation from toluene