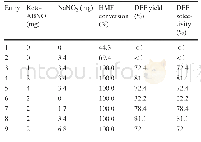
《Table 4 Product composition of diff erent adsorbent used model fuel:1-hexene (34.3%) and n-heptane
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Difunctional Adsorbents Ni/ZnO–HZSM-5 on Adsorption Desulfurization and Aromatization of Olef in Reaction》
Comparing 1-hexene(34.3%)with 1-pentene(35.0%),the conversion rate of 1-hexene(88.9%)was higher than that of1-pentene(79.4%)at the reaction time of 1.0 h on the Ni/ZnO–HZSM-5-1:1.5,as shown in Table 4.The aromatics yield of 1-hexene was lower than that of 1-pentene,whereas1-hexene exhibited better desulfurization performance than1-pentene,as shown in Fig.3.The results agree with the discussion in Section“Comparison of thiophene desulfurization and aromatization in C 5 olefi ns.”In a word,under good olefi n aromatization,thiophene desulfurization would be weakened.One of the reasons for the weaker aromatization of 1-hexene than 1-pentene is that the pore size of HZSM-5 was fi xed and measured less than 0.45 nm(as shown in Fig.7),and HZSM-5 featured very high selectivity.The fi xed pore resulted in higher molecular volume,causing the more disadvantageous generation of aromatics[29].This result also explains why the predominant aromatichydrocarbons included toluene and xylene rather than the low-molecular-weight benzene or other higher aromatics.A low benzene yield enables the quality of product to meet the strict regulation on benzene content in clean gasoline.Based on the above analysis and olefi n product analysis in Tables 2 and 4,C 5 olefi ns easily generated naphthene,whereas 1-hexene experienced diffi culty in generating the same compound,resulting in almost no naphthene in the product.These fi ndings imply that the reaction mechanism of olefi n aromatization between C 5 and C 6 may be diff erent.
| 图表编号 | XD0076872200 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.04.01 |
| 作者 | Jianzeng Du、Yonghong Li、Zhenyu Miao |
| 绘制单位 | Key Lab for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University、National Engineering Research Center for Distillation Technology、Collaborative Innovation Center of Chemical Science and Engi |
| 更多格式 | 高清、无水印(增值服务) |