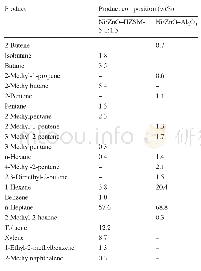
《Table 3 Product composition of1 0 0%n-heptane (wt%)》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Difunctional Adsorbents Ni/ZnO–HZSM-5 on Adsorption Desulfurization and Aromatization of Olef in Reaction》
C4:butane and 2-methyl-1-propene
The product distribution of Ni/ZnO–Al 2 O 3 was analyzed by GC–MS,as shown in Table 4.The main products were1-hexene isomerides with branched chains,accounting for 8.0%.The results imply that rearrangement reaction occurred on the active Ni sites.Based on the above discussion and experimental phenomena,we infer that the competition between sulfides and olefins did not only exist in physical adsorption on the adsorbent surface but also in subsequent chemical reaction on active Ni sites.In this process,hydrogen molecules reacted with Ni to produce H-positive and H-negative ions.Then,the H-positive ions attacked the 1-hexene adsorbed on Ni byπ-bond formation to generate carbocation[30].Next,the carbocation molecules underwent structural rearrangements and generated branched isomers on the Ni surface[31],which follows the minimum energy principle.However,while HZSM-5 was used as carrier,almost no 1-hexene isomers were present in the product.Instead,a large amount of aromatics toluene and xylene were generated,as shown in Table 4.Therefore,we speculate that in olefin aromatization,Ni played a major role in activating olefins into carbocation;then,carbocation followed oligomerization,cyclizing dehydrogenation in the pore of HZSM-5[29,32,33].On the other hand,nickel also played a key role in desulfurization via removing S atoms from the ring of thiophene.Thus,the high aromatics yield would weaken desulfurization performance because desulfurization and olefin aromatization both require the participation of active Ni sites at the same time.
| 图表编号 | XD0076871900 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.04.01 |
| 作者 | Jianzeng Du、Yonghong Li、Zhenyu Miao |
| 绘制单位 | Key Lab for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University、National Engineering Research Center for Distillation Technology、Collaborative Innovation Center of Chemical Science and Engi |
| 更多格式 | 高清、无水印(增值服务) |