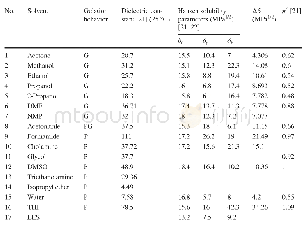
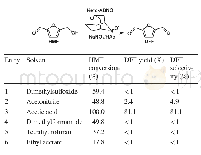
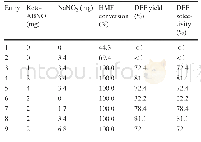
《Table 2 Aerobic oxidation of HMF to DFF with diff erent co-catalysts》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"High-Efficiency Preparation of 2,5-Diformylfuran with a Keto-ABNO Catalyst Under Mild Conditions"》
Reaction conditions:HMF(65 mg,0.5 mmol),co-catalyst(3.4 mg),keto-ABNO(2 mg),acetic acid(4 mL),O 2(10 mL/min),40°C,2 h
Based on the above results,a good yield of DFF can be achieved via the co-catalysis of NaNO 2.To gain further insights into this catalytic system,we investigated the infl uence of other commercial sodium and nitrate salts as cocatalysts on the oxidation of HMF to DFF,the results of which are summarized in Table 2.As we can see in the table,none of the sodium salts worked well(Table 2,entries 1,2,3,4).The conversions of HMF were less than 30%and DFF was almost undetectable.In contrast,the nitrate salts(Table 2,entries 5,6,8)yielded better than 90%conversions of HMF and the DFF yields were also good.It indicates that nitrate salts exhibit a signifi cant co-catalytic eff ect with ketoABNO.Of the three,NaNO 2 and Cu(NO 3)2 generated the best results with full conversions of HMF and a DFF yield of80%after reaction for 2 h in acetic acid at 40°C.Selectivity to DFF was lower when using Fe(NO 3)3 as the co-catalyst.It is easy to understand that Fe 3+exhibited a better oxidation ability than Cu 2+,which might lead to the excessive oxidation of the hydroxyl group.The HMF would overreact with other high-value furan compounds.Moreover,when we used Zn(NO 3)2 as the co-catalyst,no DFF was detected,which may be attributed to the inactivation of Zn 2+.However,the Fe 3+and Cu 2+could be easily reduced and reoxidized between diff erent valence states[28,29].In the catalytic reaction process,Fe 3+and Cu 2+are coupled with the catalyst and then react with the alcohol to form another intermediate by the release of H+.With theβ-H and reductive eliminations,the alcohol is oxidized to aldehyde as Fe 2+and Cu+are released.Then,the Fe 2+and Cu+are reoxidized to Fe 3+and Cu 2+by NO x.Based on these results,we conclude thattwo conditions must be satisfi ed for an effi cient co-catalyst.First,it should contain a lively base metal element.Second,the metal cations should be coupled with a catalyst.On this basis,NaNO 2,Cu(NO 3)2,and Fe(NO 3)3 promote the effi cient oxidation of HMF to DFF.
| 图表编号 | XD0076871400 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.04.01 |
| 作者 | Le Li、Yuefei Wang、Wei Qi、Rongxin Su、Zhimin He |
| 绘制单位 | State Key Laboratory of Chemical Engineering, School of Chemical Engineering and Technology, Tianjin University、State Key Laboratory of Chemical Engineering, School of Chemical Engineering and Technology, Tianjin University、Tianjin Key Laboratory of Membr |
| 更多格式 | 高清、无水印(增值服务) |