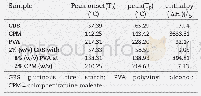
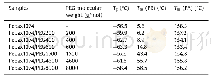
《Table 2 Thermal properties of PEF synthesized from furan route compared with HMF route》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《From Furan to High Quality Bio-based Poly(ethylene furandicarboxylate)》
a Intrinsic viscosity obtained with an Ubbelohde viscometer;b N2,the second heating scan at 10 K/min,25-280°C;c N2,heating rate 20 K/min,50-800°C
In order to evaluate the quality of PEF prepared from different FDCA(PEF-HMF and PEF-Furan,respectively),their thermal properties were investigated by DSC and TGA(Fig.6 and Table 2).It was noted that all the PEF samples,regardless of the FDCA used as starting material,showed a glass transition temperature of~87°C,a weak melting point at~214°C and a temperature of 5%weight loss at about380°C.These results were similar to those reported[42,43],and indicated that the thermal properties of PEF were not affected by the origin of FDCA.In another word,the possible trace impurities co-existing with HMF/FDCA did not influence the thermal properties of resulted PEF,though the coloration was observed.With FDCA preparation from furfural by Henkel reaction,not only the target product 2,5-FDCA was formed,but also the isomers,2,4-FDCA and 3,4-FDCA.Purification of 2,5-FDCA became much difficult and high-costing[11,28].The presence of 2,4-FDCA or 3,4-FDCA will seriously affect the thermal properties of resulted PEF,leading to a much lower glass transition temperature and melting temperature with also a weak crystallization[44].
| 图表编号 | XD0020170700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.06.01 |
| 作者 | Jing-Gang Wang、Xiao-Qing Liu、Jin Zhu |
| 绘制单位 | Ningbo Institute of Materials Technology and Engineering,Chinese Academy of Sciences、University of Chinese Academy of Sciences、Key Laboratory of Bio-based Polymeric Materials of Zhejiang Province、Ningbo Institute of Materials Technology and Engineering,Ch |
| 更多格式 | 高清、无水印(增值服务) |