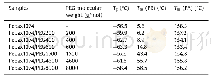
《Table 2 Thermal properties of pure PLLA and PLLA/BMCD blended polymers w ith various ratio》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Toughening of Poly (L-lactide) with Branched Multiblock Poly (ε-caprolactone)/poly (D-lactide) Copolymers》
Figure 8 show ed the DSC thermograms of PLLA and PLLA/BM CD films collected upon heating and the data of the materials w ere summarized in Table 2.As listed in Table 2,PLLA and BM CD-3%had one melting temperature at180.22℃and 180.07℃,respectively,w hile all the other films(BM CD-5%,BM CD-7%,BM CD-10%and BM CD-20%)had tw o melting temperatures at around 180℃and200℃.These melting temperatures w ere assigned to the PLLA and stereocomplex compositions,respectively.The major melting point(Tm-1)of the PLLA blends decreased from180.07℃to 176.65℃w hen BM CD concentration increased,w hich indicated that the adding of the soft segment low ered the melting point of PLLA slightly.The enthalpy(Hm)of PLLA components w ere 45.71,32.47,30.69,22.72,25.61 and26.55 J/g for pure PLLA,BM CD-3%,BM CD-5%,BM CD-7%,BM CD-10%and BM CD-20%,respectively.The second melting point(Tm-2)of the PLLA blerds increased from194.49℃to 205.80℃w hen BM CD concentration increaced.The enthalpy Hm-2 of stereocomplex components w ere 0.67,2.59,4.34,and 9.08 J/g for BM CD-5%,BM CD-7%,BM CD-10%and BM CD-20%respectively,indicating that more stereocomplex formed as the BM CD contents increased.The enthalpy of PLLA components decreased at first and then rose as the BM CD contents increased.A possible explanation of theenthalpydifferencesw asthatthestereocomplex crystallization(SC)formed first in the blending,and the SC hindered the formation of PLLA homocrystallization[32].As the BM CD content increased,the stereocrystals acted as nucleating agents to promote the crystallization of PLLA[33].
| 图表编号 | XD0014950800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.10.31 |
| 作者 | 常悦、陈支泽、杨一奇 |
| 绘制单位 | College of Chemistry,Chemical Engineering and Biotechnology,Donghua University、College of Chemistry,Chemical Engineering and Biotechnology,Donghua University、Department of Textiles,Merchandising and Fashion Design,University of Nebraska-Lincoln、Department |
| 更多格式 | 高清、无水印(增值服务) |