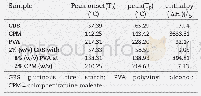
《Table 2 Thermal properties of FPET-1, CFPET-1 and CFPET-2in air》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"Synthesis and Characterization of Fluorinated Polyurethane Containing Carborane in the Main Chain: Thermal, Mechanical and Chemical Resistance Properties"》
In order to evaluate the initial decomposition behavior of polymers,T5 is defined as the temperature at which the resin loses 5%of its original weight,and T10 is defined as the temperature at which the resin loses 10%of its original weight.T5 and T10 values and the char yields(Yc,at 800°C)of the three fluorinated polyesters(CFPET-1,CFPET-2 and FPET-1)are summarized in Table 2.Obviously,T5 values for CFPET-1,CFPET-2 and FPET-1 are 285,229 and161°C,respectively.And T10 values for them are 365,328and 270°C,respectively.These data indicate that the carborane-containing fluorinated polyesters(CFPET-1 and CFPET-2)have higher thermal stability than the carborane-free fluorinated polyester(FPET-1).Meanwhile,the ultimate char yields(Yc)of carborane-containing fluorinated polyesters CFPET-1 and CFPET-2 at 800°C are32.1%and 22.2%,respectively.Such data are superior to the carborane-free fluorinated polyester FPET-1 with a low char yield of only 6.0%under the same conditions.The high char yield and decomposition temperature of carborane-containing polyesters indicate that the carborane group can effectively reduce the degradation rate of carborane-containing polyesters.On the other hand,T5 and T10 values and the char yield(800°C)of CFPET-1 are higher than those of CFPET-2.These results demonstrate that the length of carbon chain connecting to the carborane group relates to the thermal resistance of carborane-containing polyester.The shorter the carbon chain lengths are,the better the thermal resistance of carborane-containing polyester will be.The reason may be that the fluorinated polyester with the shorter carbon chain has the stronger steric effect than that with longer carbon chain,which impedes the movement of molecules at high temperature.The results also coincide with our previous work[37].
| 图表编号 | XD0020167300 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.01.01 |
| 作者 | Ning Li、Fang-Lei Zeng、Yu Wang、De-Zhi Qu、Chun Zhang、Juan Li、Jin-Zhao Huo、Yong-Ping Bai |
| 绘制单位 | School of Chemistry and Chemical Engineering, Harbin Institute of Technology、School of Materials Science & Engineering, Beijing Institute of Technology、School of Chemistry and Chemical Engineering, Harbin Institute of Technology、School of Chemistry and Ch |
| 更多格式 | 高清、无水印(增值服务) |