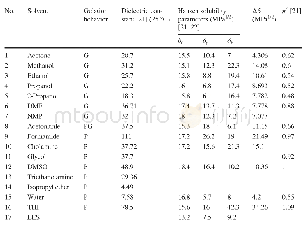
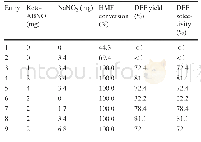
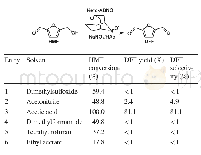
《Table 1 Aerobic oxidation of HMF to DFF in various solvents》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"High-Efficiency Preparation of 2,5-Diformylfuran with a Keto-ABNO Catalyst Under Mild Conditions"》
Reaction conditions:HMF(65 mg,0.5 mmol),NaNO 2(3.5 mg,0.025 mmol),keto-ABNO(2 mg),solvent(4 mL),O 2(10 mL/min),40°C,2 h
We used keto-ABNO and NaNO 2 to catalyze the oxidation of HMF to DFF(Table 1).In this system,keto-ABNO serves as the catalyst and NaNO 2 as the co-catalyst.As the solvent polarity,dielectric constant,steric hindrance,and acid–base properties play decisive roles in reactions[27],we fi rst investigated the infl uence of various solvents on the catalytic reaction.Table 1 shows the results,in which we can see that reaction in acetic acid proved to be the best,with a full conversion of HMF and 81.1%selectivity of DFF through the catalysis of keto-ABNO and NaNO 2 for 2 h at 40°C.When using other solvents,i.e.,acetonitrile,dimethylsulfoxide,dimethylformamide,tetrahydrofuran,and ethyl acetate,we detected almost no DFF product.In studies,acetonitrile has been frequently used when ABNO is the catalyst.However,in our catalytic system,the yield of DFF in acetonitrile was very low.This distinct diff erence might be attributed to the use of a diff erent co-catalyst from that had been used in previous work.In the keto-ABNO and NaNO 2 catalytic system,the NO x sources serve as an intermediate of the electron transfer,which is crucial for the effi cient catalysis of HMF.In the study of Wang et al.[28],NO x was generated by Fe 3+and NO 3-.However,in our catalytic system,NO x sources are generated from NO 2-and H+.In the absence of H+,no NO x would form and thus the yield of DFF would be negligible.
| 图表编号 | XD0076871300 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.04.01 |
| 作者 | Le Li、Yuefei Wang、Wei Qi、Rongxin Su、Zhimin He |
| 绘制单位 | State Key Laboratory of Chemical Engineering, School of Chemical Engineering and Technology, Tianjin University、State Key Laboratory of Chemical Engineering, School of Chemical Engineering and Technology, Tianjin University、Tianjin Key Laboratory of Membr |
| 更多格式 | 高清、无水印(增值服务) |