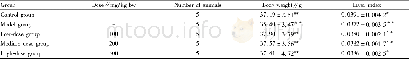
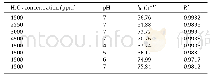
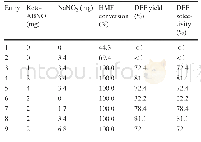
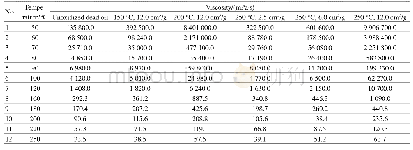
《Table 3 Oxidation rates of benzyl alcohol and benzaldehyde related to system pressure and catalyst
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Kinetics and Mechanism Modeling of Liquid-Phase Toluene Oxidation to Benzaldehyde Catalyzed by Mn–Mo Oxide》
Several other reports[30–33]showed that the oxidation of benzyl alcohol to benzaldehyde was proportional to the benzyl alcohol concentration,oxidant,and quantity of catalyst,respectively.In our work,it was discovered that theconcentration ratio of intermediate benzyl alcohol to benzaldehyde remained constant during the initial oxidation stage of 0.5–1.5 h(0.47–0.49 at 413 K,0.50–0.52 at 423 K,0.54–0.57 at 423 K,and 0.59–0.61 at 443 K.See Table S2).This phenomenon demonstrates that the oxidation of toluene is possibly a parallel reaction due to the constant ratio of the two diff erent oxidation products[34].Therefore,we fi rst assumed that the initial generation rates of benzyl alcohol and benzaldehyde are both fi rst order to toluene.The consideration of oxygen partial pressure and surface area of catalyst was suspended due to the fi xed 1.0 MPa and 2.0 g catalyst.Finally,based on the fi rst-order parallel-consecutive law,the generation of benzaldehyde is given by Eq.(6):
| 图表编号 | XD0042637200 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.01 |
| 作者 | Wang Li、Qingjun Zhang、Aiwu Zeng |
| 绘制单位 | School of Chemical Engineering and Technology, Tianjin University、School of Chemical Engineering and Technology, Tianjin University、School of Chemical Engineering and Technology, Tianjin University |
| 更多格式 | 高清、无水印(增值服务) |