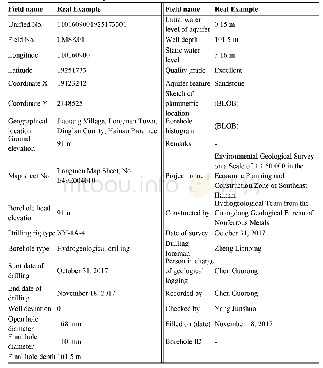
《Table 1 Concentration of alkalis oxides in the raw mixtures and C3S》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Influence of Alkalis Doping on the Hydration Reactivities of Tricalcium Silicate》
Note:Free lime content of control in the first cycle and second is f-CaO=0.27 and f-CaO=0.18,respectivelyΔf-CaO=Difference of CaO content between the doped C3S and pure C3S Ca/M:molar ratio of change of Ca-ions to alkali ions retained in the final sample
In order to produce C3S solid solutions without any by-product,the amount of alkalis added should be chosen according to the solid solution limit.But to counteract the evaporation of alkalis,a certain excess of alkali oxides was added.The starting material C3S was further mixed with Li2CO3,Na2CO3·10H2O,and K2CO3in various molar ratios,respectively(Table 1).The mixed powder was pressed into pellets and heated from1 250℃at a rate of 4℃/min up to 1 600℃,then kept at 1 600℃for 3 h.The cycle of grinding and firing was repeated until a constant free lime concentration was reached,indicating an equilibrium state.The same process was also carried out on the pure C3S and its final free lime was less than 0.2%.After the synthesis,all samples were ground under the same conditions to about 340 m2/kg(Blaine).
| 图表编号 | XD0043326600 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.01 |
| 作者 | 任雪红、张文生、YE Jiayuan、SHEN Xiaodong、WANG Fazhou |
| 绘制单位 | State Key Laboratory of Green Building Materials, China Building Materials Academy、State Key Laboratory of Green Building Materials, China Building Materials Academy、State Key Laboratory of Green Building Materials, China Building Materials Academy、The Sy |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 1 Concentration of alkalis oxides in the raw mixtures and C3S”的人还看了
-
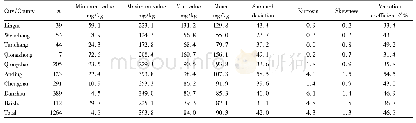
- Table 3 Distribution characteristics of soil alkali-hydrolyzable nitrogen content in the Nandu River Basin of Hainan Pro