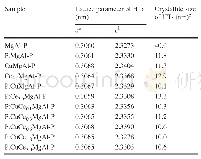
《Table 2 The shift of the lattice parameters of tricalcium silicate by incorporation of alkalis》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Influence of Alkalis Doping on the Hydration Reactivities of Tricalcium Silicate》
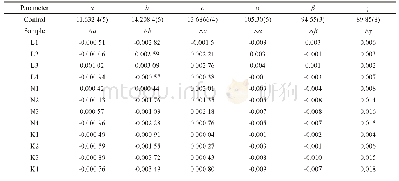
The characteristic of the selected angular windows(31.5-33.5°and 51°-53°)appearing in the XRD patterns is the same for all the samples,and concurs with the characteristic of T1 form C3S,indicating that the samples with different alkali contents but the same polymorphic form were produced.Based on the X-ray diffraction data,the crystal structures of the samples were refined by the Rietveld method.The internal T1 structure was fitted to that of Golovastikov et al(triclinic C3S,ICSD codes:4331)[18].Fig.1 shows the refinement plots of data for pure C3S and a feature inset of the fingerprint regions unique to T1 form.On casual inspection of the residual Rietveld plots in Fig.1,the fits appear excellent.After refinement,the cell parameters were extracted.Changes of lattice parameters a,b,c andα,β,γof doped C3S in comparison with the pure C3S are given in Table 2.Changes in the crystal structure of C3S could mainly be monitored by changes in parameters b,c andγ.Parameter b mainly exhibits a slight decrease,and the increase of parameter c andγwas detected.Changes in lattice parameters seem to vary irregularly with the increasing amount of both Li and Na.However,more changes were observed with the increasing of K.Similar changes in the crystal strain are also observed(Table 3).This can be linked to the different substitution types of alkalis.K can only replace Ca,a greater change in structure will be caused by an increase in K content,and the crystal strain increases correspondingly.However,this is not the case for Li and Na.Li and Na can occupy interstitial voids(Li also may replace Si),and a lattice contraction resulting from the substitution for Ca can be counteracted by a lattice expansion resulting from alkali interstitials,therefore,irregular changes in the lattice parameters are observed.These results are in fair agreement with the role of alkalis in C3S[5].
| 图表编号 | XD0043326700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.01 |
| 作者 | 任雪红、张文生、YE Jiayuan、SHEN Xiaodong、WANG Fazhou |
| 绘制单位 | State Key Laboratory of Green Building Materials, China Building Materials Academy、State Key Laboratory of Green Building Materials, China Building Materials Academy、State Key Laboratory of Green Building Materials, China Building Materials Academy、The Sy |
| 更多格式 | 高清、无水印(增值服务) |