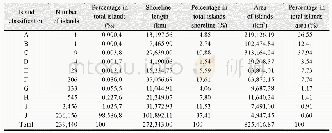
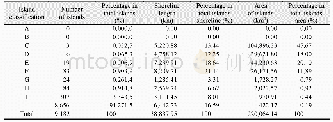
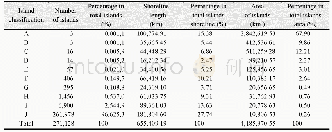
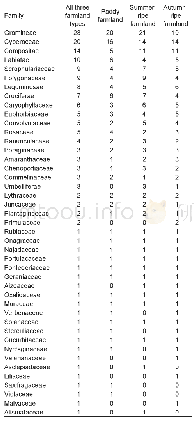
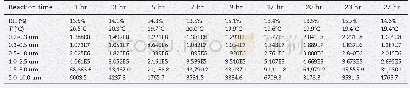
《Table 5–Variation in particle number concentration induced by oxidation (sulfur dioxide) .》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Potential factors and mechanism of particulate matters explosive increase induced by free radicals oxidation》
Sulfur dioxide is an important atmospheric pollutant that causes acid rain and generates sulfate;to some extent,it directly damages the ecosystem and threatens the survival of humanity.As a certain amount of SO2was emitted in the smog chamber,the PNC produced two peak values with ozone,as shown in Table 5.During the initial stage of the reaction,the PNC decreased over time and then gradually increased until the highest value emerged.From then on,the PNC slowly decreased to near the background level because the chain reaction was terminated and energy was lost.These results indicate that a significant amount of time is needed to nucleate and accumulate large amounts of ultrafine particles before particulate matter explosive increase occurred.We attributed the first peak to the high concentration of ozone,which triggered new particle formation.By contrast,the second peak resulted from long-term accumulation because the half-life of ozone is only0.5 hr.Unfortunately,the ultrafine particle size was below the instrument detection limit.However,SO2has been proven to play a considerable role in the formation of particulate matter and should attract more attention in the future.
| 图表编号 | XD0033531100 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.07.15 |
| 作者 | Guoying Wang、Shiming Jia、Xiuli Niu、Haoqi Tian、Yanrong Liu、Zhong Xie、Chao Liu、Yucan Dong、Ying Su、Jianglei Yu、Gaofeng Shi、Xuefu Chen、Lan Li、Peng Zhang |
| 绘制单位 | School of Petrochemical Engineering, Lanzhou University of Technology、School of Petrochemical Engineering, Lanzhou University of Technology、Gansu Province Food Inspection Institute、School of Petrochemical Engineering, Lanzhou University of Technology、Scho |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 5–Variation in particle number concentration induced by oxidation (sulfur dioxide) .”的人还看了
-

- Table 16 Number,shoreline length,and area of islands in South America by area classification in 2015