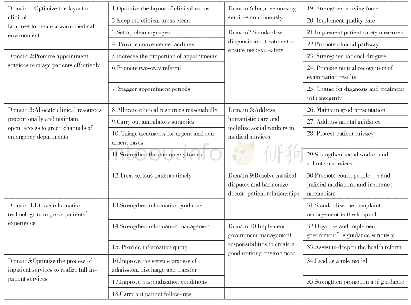
《Table 1.The composition and specifications of the prepared catalysts.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Fischer–Tropsch synthesis using Co and Co-Ru bifunctional nanocatalyst supported on carbon nanotube prepared via chemical reduction method》
*Surface displacement synthesis.**Impregnation synthesis.
In the present investigation,we synthesized the Co-Ru nanoparticles supported over functionalized CNTs through a surface displacement reaction.Fig.1 shows the schematic view of the synthesis route of Co-Ru nanoparticles over CNTs.Hence,we employed a two-step reduction route for the reduction of both Co Cl2·6H2O and RuCl3using NaBH4and N2H4,respectively.Thus in the first step,through the reaction of CoCl2·6H2O with the NaBH4solution as reducing agent,the cobalt Nano crystallites are formed at CNTs surface.500 mg of the acid-treated CNTs was dispersed in 100 mL water-ethanol(volume ratio=1:1)solution for 30 min using ultrasonic.Afterward,202 mg of the CoCl2·6H2O aqueous solution was added to the suspension and stirred for 2 h.Then,a solution of NaBH4as the reducing agent(4 mg in 10 mL of 0.3 M NaOH solution),was added drop-wise to the solution under stirring at room temperature.Following 10 h,the Co nanocrystals were deposited at CNTs support so that a Co/CNTs compound was formed.In the second step,the obtained suspension containing Co/CNTs nanoparticles was heated to 453 K.Afterwards,10 mL aqueous solution of Ru Cl3was added drop-wise by stirring into suspension.It is necessary to mention that in this synthesis stage,a reflux flow was performed to maintain the suspension at 453 K.Following 1 h,we added 4 milliliters of N2H4solution(as a second reducing agent)drop-wise under stirring for 6 h so that Ru was precipitated and deposited at Co/CNTs particles.Then,the Co/Ru/CNTs compound was filtrated using a filtration paper and washed out with DDI water and ethanol for several times.The prepared solid sample(catalyst)was dried in a vacuum oven at 383 K for 8 h.To remove impurities,the prepared catalyst supported over CNTs was calcined at 623 K for 5 h under flow of argon(Ar)to comply with the characteristic conditions of CNTs[38,39].Similarly to synthesis the Co/Ru/CNTs catalyst,the nanoparticle mixture of Co/CNTs was washed several times by ethanol and DDI water,then,dried at 383 K and finally calcined under argon flow at 623 K for 5 h.It is worth mentioning that the Co content was fixed at 10 wt%for all the synthesized catalysts.In addition,in the present study,the two Co/γ-Al2O3and Co-Ru/γ-Al2O3catalysts with 0 and 1 wt%of Ru loading,respectively,and 10 wt%of Co were prepared using the same synthesis procedure.These two alumina-supported catalysts were used in order to compare their results to the other Cosupported catalysts.Finally for comparison purposes,the two different sets of catalysts(Co/CNTs and Co-Ru/CNTs)were synthesized by traditional impregnation technique using aqueous solutions of Co Cl2·6H2O and Ru Cl3.The prepared catalysts nomenclature and their compositions are summarized in Table 1.As seen,the Ru and Co contents of the calcined catalysts are fairly similar and close to the theoretical metal contents.
| 图表编号 | XD0042894200 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.01 |
| 作者 | Jafar Shariati、Ali Haghtalab、Amir Mosayebi |
| 绘制单位 | Department of Chemical Engineering, Tarbiat Modares University、Department of Chemical Engineering, Tarbiat Modares University、Department of Chemical Engineering, Tafresh University |
| 更多格式 | 高清、无水印(增值服务) |