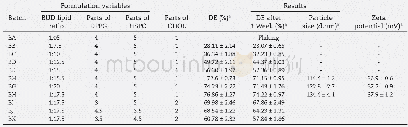
《Table 1–The composition of liposome, invasome and transfersome formulations.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"Vesicular carriers containing phenylethyl resorcinol for topical delivery system; liposomes, transfersomes and invasomes"》
Preparation of PR-loaded transfersomes and invasomes was followed and modified from the method of Limsuwan et al.[13].The PR in the formulations was fixed as 0.5%(w/v).The0.5%(w/v)CHOL,3%(w/v)SPC and water up to 100%(v/v)were the main composition of liposomes.The skin enhancers were varied in terms of concentrations and ratios as shown in the Table 1.The invasome formulations used fenchone,citral and d-limonene mixed with 10%(v/v)ethanol as skin enhancers.The transfersome formulations used Tween 80,Tween 20,Span 80,Span 20 and SDC as skin enhancers.All compositions were prepared by thin-film hydration method as follows:First,the oil phase which included SPC,CHOL,PR and skin enhancer were dissolved in absolute ethanol.The aqueous phase used was water for liposomes and transfersomes;a hydro-ethanolic solution consisting of water and 10%(v/v)absolute ethanol for invasomes.Second,the oil phase and aqueous phase were separately sonicated at 60°C for30 min until homogeneity.Then,the absolute ethanol was removed from oil phase via evaporation by a rotary evaporator(Model Eyela N-1000 series,Tokyo Rikakikai Co.,Ltd.,Japan)while the other components formed the thin lipid film.Afterward,the lipid film was hydrated with 10 ml of aqueous phase,followed by shaking for 5 min.Finally,the mixtures were sonicated at 60°C for 30 min to obtain the complete formulations.
| 图表编号 | XD00598700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.09.01 |
| 作者 | Thanaporn Amnuaikit、Tunyaluk Limsuwan、Pasarat Khongkow、Prapaporna Boonme |
| 绘制单位 | Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Prince of Songkla University、Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Prince of Songkla University、Institute of Biomedical Engineering, Facult |
| 更多格式 | 高清、无水印(增值服务) |