《Table 3–Acidity (pH) of suspension used for KPW and BKPW composite samples preparation and after gl
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《In situ synthesis of potassium tungstophosphate supported on BEA zeolite and perspective application for pesticide removal》
Langmuir and Freundlich adsorption isotherms are often used to model adsorption behavior on the subject of pollutants removal.Fitting results found for these two isotherm models and maximum recorded adsorbed amount of pesticide(qe)are presented in Table 2.Adsorption isotherms for all investigated samples obtained from two models(lines)and experimental adsorption values(squares)are given in Fig.7.The variety of used adsorbents in environmental applications such as soil particles or different composite materials usually have non-homogeneous surfaces,with energetically diverse active sites which involve Freundlich isotherm model for pollutants adsorption.
| 图表编号 | XD0033529400 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.07.15 |
| 作者 | Bojana Nedi Vasiljevi、Milena Obradovi、Danica Bajuk-Bogdanovi、Maja Milojevi-Raki、Zoran Jovanovi、Nemanja Gavrilov、Ivanka Holclajtner-Antunovi |
| 绘制单位 | Faculty of Physical Chemistry, University of Belgrade、Faculty of Physical Chemistry, University of Belgrade、Faculty of Physical Chemistry, University of Belgrade、Faculty of Physical Chemistry, University of Belgrade、Laboratory of Physics, Vina Institute o |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 3–Acidity (pH) of suspension used for KPW and BKPW composite samples preparation and after glyphosate adsorption a”的人还看了
-

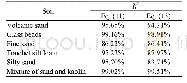
- Table 1Chemical analysis of soils under organic and chemical fertilizing conditions used for growth of C.sativum plants.
-
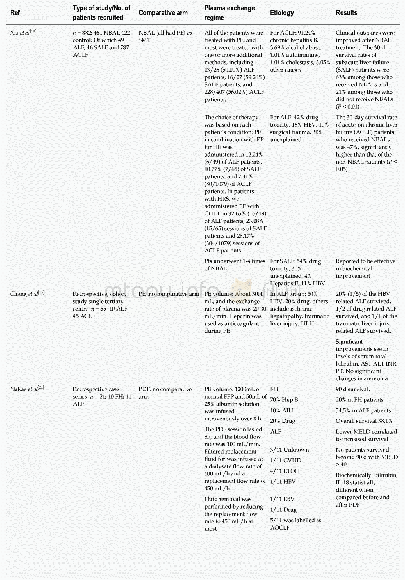
- Table 5 Studies included for use of plasmapheresis in acute liver failure and acute-on-chronic liver failure in adults
-

- Table 5 Studies included for use of plasmapheresis in acute liver failure and acute-on-chronic liver failure in adults