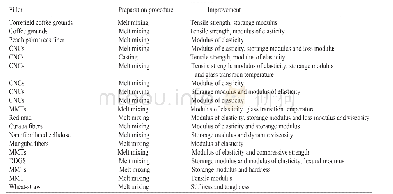
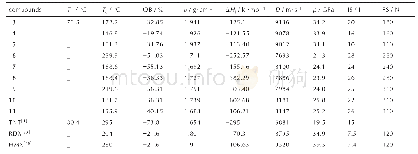
《Table 2The physicochemical properties of 3-11 compared with trinitrotoluene (TNT) , 1, 3, 5trinitro
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"5,5′-二硝胺基-2,2′-联-1,3,4-噁二唑含能离子盐的合成及性能(英文)"》
Note:Tmis melting point.Tdis decomposition temperature from DSC(5℃·min-1).OB is oxygen balance(%)for CaHbNcOd,and OB%=1600×(d2ab/2)/Mw(based on carbon dioxide).ρis density measured using a gas pycnometer(25℃).ΔHfis calculated molar entha
The enthalpy of formation(HOF)is essential for calculating the detonation performance.The heats of formation of the synthesized compounds 3-11 were calculated based on appropriate isodesmic reac?tions.Calculations were carried out using the Gauss?ian 09 program suite.The geometry optimization of the structures and frequency analyses were carried out using the B3LYP functional with the 6?311+G**basis set.All of the optimized structures were charac?terized by true local energy minima on the potential energy surface without imaginary frequencies.The results are summarized in Table 2,the enthalpy of for?mation ranging from-0.671(6)kJ·g-1to 1.143 kJ·g-1(10).With the exception of 3,4 and 6,the energet?ic salts possess positive heats of formation,which are higher than TNT(-1.30 kJ·g-1)and RDX(0.32 kJ·g-1)due to a large number of C—N and N—N bonds,andcompound 10 has the highest value of 1.143 kJ·g-1.
| 图表编号 | XD0026273200 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.11.25 |
| 作者 | 熊华林、杨红伟、程广斌 |
| 绘制单位 | 南京理工大学化工学院、南京理工大学化工学院、南京理工大学化工学院 |
| 更多格式 | 高清、无水印(增值服务) |