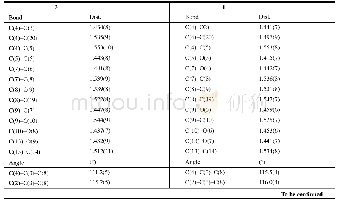
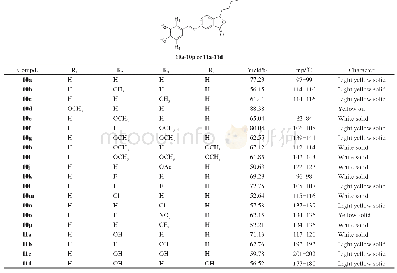
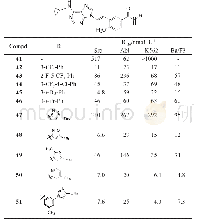
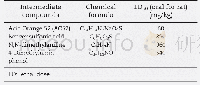
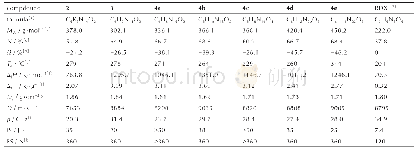
《Table 1Physicochemical and energetic properties of compounds 2, 3, and 4a-4e compared to RDX》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《高热稳定和低感度多环类含能材料的设计与合成(英文)》
Note:[a]molecular formula.[b]molecular weight.[c]nitrogen content.[d]OB for CaHbOcNd,1600(cab/2)/MW(based on CO).[e]decomposition temperature (onset temperature at a heating rate of 10℃·min-1.[f]heat of formation.[g]density measured using a gas p
As shown in Table 1,the physicochemical and energetic properties of all as?prepared energetic com?pounds were determined by both calculations and measurements.Most of these energetic compounds(except compound 2)had high nitrogen contents ranging from 60.9%(4c)to 68.8%(4b),exceeding that of RDX.The CO?based oxygen balances of these compounds varied from-20.7 to-46.2 due to existence of only two oxygen atoms in energetic ion?ic parent structure.The thermal stabilities of neutral compound 3 and its energetic salts were investigated by differential scanning calorimetry(DSC)at heat?ing rate of 10℃·min-1.Except for energetic salt 4c(229℃),compound 3 and other salts all exhibited high thermostability values with decomposition tem?peratures above 260℃.These values were obvious?ly superior to those of common explosives RDX(204℃),CL?20(215℃),and TKX?50(221℃).Especially,the decomposition temperature of salt 4e reached as high as 314℃,indicating its great poten?tial as heat?resistant explosive.
| 图表编号 | XD0026273000 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.11.25 |
| 作者 | 李卫、王毅、亓秀娟、宋思维、王康才、靳云鹤、刘天林、张庆华 |
| 绘制单位 | 中国工程物理研究院化工材料研究所、中国工程物理研究院化工材料研究所、西南科技大学材料科学与工程学院、中国工程物理研究院化工材料研究所、中国工程物理研究院化工材料研究所、中国工程物理研究院化工材料研究所、中国工程物理研究院化工材料研究所、中国工程物理研究院化工材料研究所 |
| 更多格式 | 高清、无水印(增值服务) |