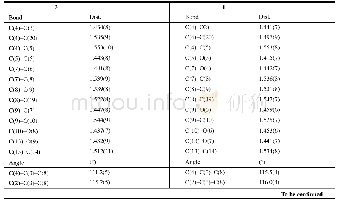
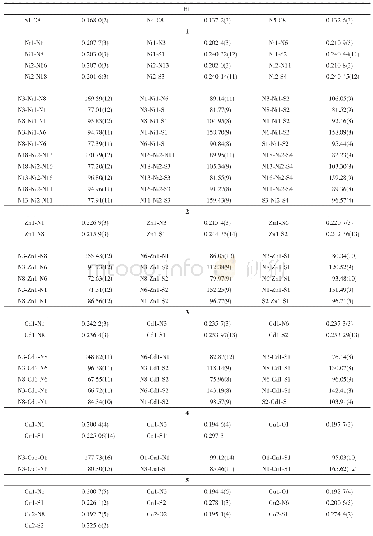
《Table 2.Selected Bond Lengths(),Bond Angles(°)and Torsion Angles(°)of Compounds 3 and 4》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Crystal Studies and Antitumor Activities of Novel D-seco-taxoids Derived from 1-Deoxybaccatin Ⅵ》
The suitable colorless single crystals of the title compounds were obtained by slow diffusion in EtOAc and petroleum ether.A summary of the crystallographic information is given in Table 1.The selected bond distances and bond angles are listed in Table 2.The selected crystal was mounted on an ENRAF-NONIUS CAD4 diffractometer.Diffraction data were measured at 293(2)K using graphitemonochromatic Mo Kα(λ=0.071073 nm)radiation.The structure was solved by direct methods using SHELXS-97,and refined by full-matrix least-squares on F2,SHELXL-97.All non-hydrogen atoms were refined anisotropically,and all hydrogen atoms were placed in calculated positions and refined with a'rider'scheme allowing for the rotation of methyl(AFIX 137)and hydroxyl(AFIX 147)groups.It should be noted that there are two pores,338?3,in the unit cell of compound 3 which are caused by solvent molecules.
| 图表编号 | XD00102429800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.09.01 |
| 作者 | 谢程虎、王佃龙、崔永梅、林海霞 |
| 绘制单位 | Department of Chemistry, College of Sciences, Shanghai University、Department of Chemistry, College of Sciences, Shanghai University、Department of Chemistry, College of Sciences, Shanghai University、Department of Chemistry, College of Sciences, Shanghai Un |
| 更多格式 | 高清、无水印(增值服务) |


![表2 化合物主要键长 (nm) 和键角 (°) 数据Tab.2 Selected Bond lengths[]and angles[°]for complex](http://bookimg.mtoou.info/tubiao/gif/MYSF201902009_05601.gif)
![表2 化合物主要键长 (nm) 和键角 (°) 数据Tab.2 Selected Bond lengths[]and angles[°]for complex](http://bookimg.mtoou.info/tubiao/gif/MYSF201902009_05600.gif)
