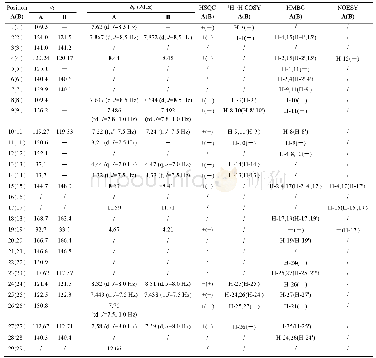
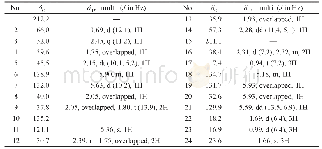
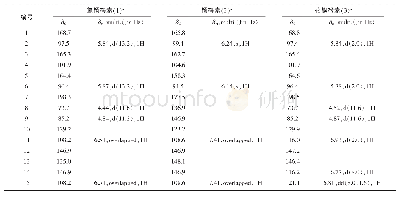
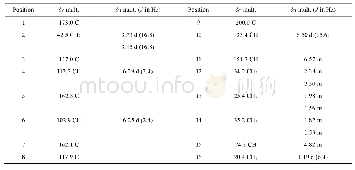
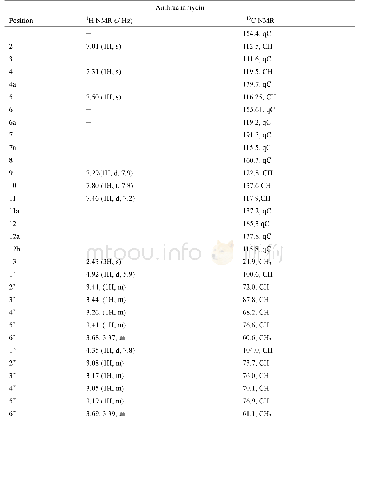
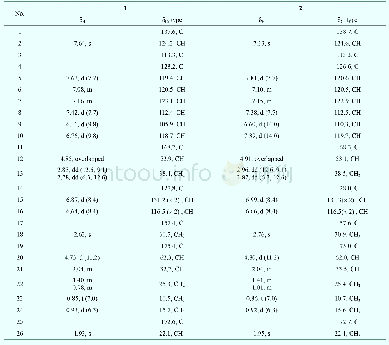
《Table 1 1H NMR (700 MHz) and 13C NMR (175 MHz) data of compounds 1 and 2 in CD3OD (J in Hz)》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Peptides and polyketides isolated from the marine sponge-derived fungus Aspergillus terreus SCSIO 41008》
Compound 1(Fig.1)was obtained as colorless oil.It gave a molecular formula of C28H34N4O4 as determined by the quasi-molecular ion peak at m/z 513.2482[M+Na]+(Calcd.513.2478)observed from the HR-ESI-MS data.The 1H NMR spectrum(Table 1)along with HSQC experiment of 1 showed eleven aromatic or olefinic(δH 6.16?7.63),three methinic[δH4.85(overlapped,H-12),4.73(d,J=11.2 Hz,H-20),2.04(m,H-21)],two methylenic[δH 2.83(dd,J=9.1,13.3 Hz,H-13a),2.78(dd,J=6.3,13.3 Hz,H-13b),1.40(m,H-22a),0.98(m,H-22b)],and four methyl[two singlets(δH 2.63 for H3-18 andδH 1.93 for H3-26),one doublet(δH 0.93,d,J=6.3 Hz,H3-24),and one triplet(δH 0.85,t,J=6.3 Hz,H3-23)]proton signals.Apart from the above 20 corresponding hydrogen-bearing carbons,eight carbons remained in the 13C NMR spectrum,including three carbonyls and five olefinics(one oxygenated).
| 图表编号 | XD0062384100 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.20 |
| 作者 | LUO Xiao-Wei、LIN Yun、LU Yong-Jun、ZHOU Xue-Feng、LIU Yong-Hong |
| 绘制单位 | CAS Key Laboratory of Tropical Marine Bio-resources and Ecology, Guangdong Key Laboratory of Marine Materia Medica,South China Sea Institute of Oceanology, Chinese Academy of Sciences、University of Chinese Academy of Sciences、School of Life Sciences, Sun |
| 更多格式 | 高清、无水印(增值服务) |