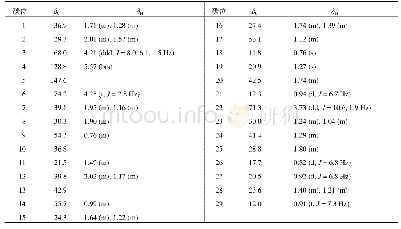
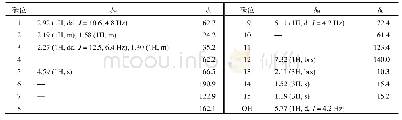
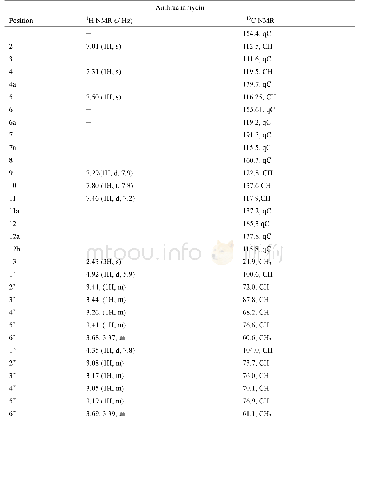
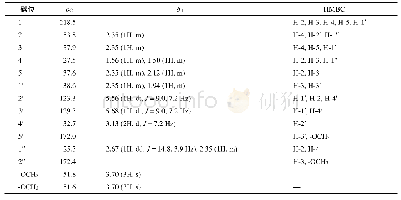
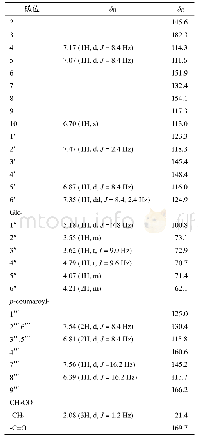
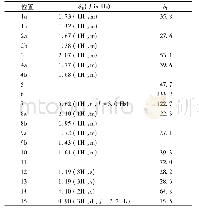
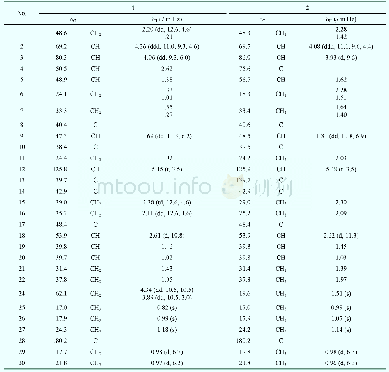
《Table 1 1H (600 MHz) and 13C NMR (150 MHz) data of compounds 1 and 2 (in C5D5N, J in Hz)》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Two new ursane-type nortriterpenes from Lonicera macranthoides and their iNOS-inhibitory activities》
Multiplets and or overlapped signals are reported without designating multiplicity.
Compound 1 was obtained as a white amorphous powder with[?]D25+15.24(c 0.5,CH3OH).The HR-ESI-MS exhibited a peak at m/z 473.3259[M-H]–(Calc.473.3267),corresponding to a molecular formula C29H46O5 with seven degrees of unsaturation.IR absorption bands at 3412 and 1691 cm–1indicated the presence of hydroxyl and ketone groups.The 1H NMR(600 MHz,in C5D5N)of 1 displayed an olefinic proton atδH 5.45(1H,t,J=3.5 Hz),five methyls[δH 1.18(3H,s),0.99(3H,s),0.98(3H,d,J=6.7 Hz),0.97(3H,d,J=6.2 Hz),and 0.82(3H,s)],an oxygenated methylene[δH 4.34(1H,dd,J=10.5,10.5 Hz),3.89(1H,dd,J=10.5,2.0 Hz)],and two oxygenated methines[δH 4.26(1H,ddd,J=11.0,9.3,6.0 Hz),4.06(1H,dd,J=9.3,6.0 Hz)].In the 13C NMR(DEPT 135)spectra,29 carbon signals could be revealed as five methyls,nine methylenes(an oxygenated atδC 62.1),nine methines(two oxygenated atδC 80.3 and 69.2),and six quaternary carbons(a carbonyl atδC 180.2,Table 1).Such evidence mentioned above suggested that 1 might be a highly oxygenated nortriterpenoid.An analysis of key COSY and HMBC correlations were used to establish the planar structure of compound 1,as shown in Fig.2.The spin system of H2-1/H-2/H-3/H-4(H2-24)/H-5/H2-6/H2-7 deduced from 1H-1H COSY experiment,together with the correlations of H3-25 to C-1,C-5,C-9,and C-10,and of H3-26 to C-7,C-8,C-9,and C-14,confirmed the linkage and assignment of rings A and B.The substructure of ring C was confirmed by the COSY correlations of H-9/H2-11/H-12,and HMBC correlations of H3-27 to C-8,C-13,C-14,and C-15,and of H-12 to C-13,and C-14.Similarly,rings D and E were established by the COSY correlations of H2-15/H2-16,and H-18/H-19(H3-29)/H-20(H3-30)/H2-21/H2-22,together with the HMBC correlation peaks of H-18/C-13,H2-16/C-17,18,22,28,and H2-22/C-16,17,18,28.
| 图表编号 | XD0047580900 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.20 |
| 作者 | MEI Yu-Dan、ZHANG Nan、ZHANG Wei-Yang、TANG Jin-Shan、ZHOU Hua、YU Yang、YAO Xin-Sheng |
| 绘制单位 | Institute of Traditional Chinese Medicine & Natural Products,College of Pharmacy and Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research,Jinan University、XiangXue Academician workstation,Xiangxue Pharmaceutical |
| 更多格式 | 高清、无水印(增值服务) |